The Experience of the SPS Committe in Developing










- Slides: 11

The Experience of the SPS Committe in Developing and Implementing Guidelines on Equivalence Marième Fall Agriculture and Commodities Division 8 November 2011

Equivalence – Article 4 of the SPS Agreement If the exporting country objectively demonstrates that its measures achieve the same ALOP as the importing country Members shall Accept SPS measures of other Members as equivalent 2

Equivalence – Article 4 of the SPS Agreement 1. Members shall accept the sanitary or phytosanitary measures of other Members as equivalent, even if these measures differ from their own or from those used by other Members trading in the same product, if the exporting Member objectively demonstrates to the importing Member that its measures achieve the importing Member's appropriate level of sanitary or phytosanitary protection. For this purpose, reasonable access shall be given, upon request, to the importing Member for inspection, testing and other relevant procedures. 2. Members shall, upon request, enter into consultations with the aim of achieving bilateral and multilateral agreements on recognition of the equivalence of specified sanitary or phytosanitary measures.

Why develop guidelines on equivalence? • Operationalize the provisions of Article 4 (LDCs, Developing Countries - problems in implementation) • Required ALOP can be reached in different ways! Equivalence contributes to … - Flexibility - Regulatory diversity -Entails mutual learning, policy innovations - Market access in situations where harmonization is not feasible due to political, epidemiological etc. reasons

Equivalence – Committee Guidelines (G/SPS/19/Rev. 2) Can be requested for individual measures / products or whole systems Importing country should identify risks and explain its ALOP Importing country should provide its risk assessment or technical justification for its own measure Respond to requests within 6 months 5

Equivalence – Committee Guidelines (G/SPS/19/Rev. 2) Take into account current history of trade Provide technical assistance to developing countries who request recognition of equivalence Notify SPS Committee when equivalence is recognized SPS Committee to follow recognition agreements, bilateral arrangements Encourage Codex, OIE and IPPC to develop guidelines for equivalence 6

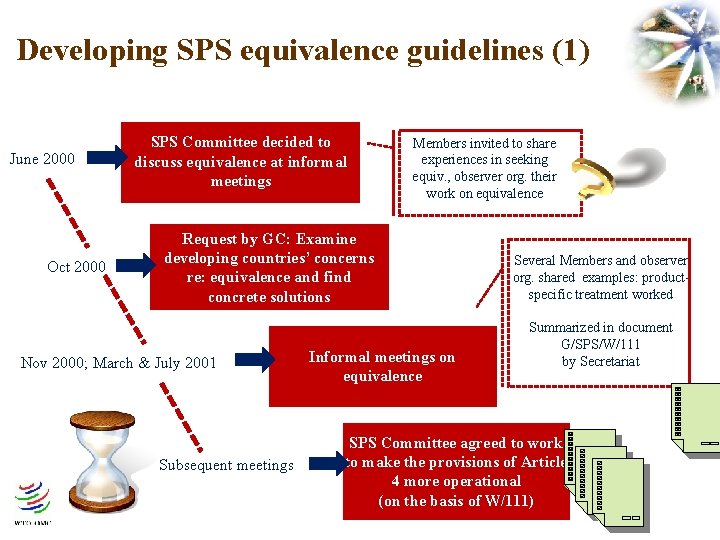
Developing SPS equivalence guidelines (1) June 2000 Oct 2000 SPS Committee decided to discuss equivalence at informal meetings Members invited to share experiences in seeking equiv. , observer org. their work on equivalence Request by GC: Examine developing countries’ concerns re: equivalence and find concrete solutions Nov 2000; March & July 2001 Subsequent meetings Informal meetings on equivalence Several Members and observer org. shared examples: productspecific treatment worked Summarized in document G/SPS/W/111 by Secretariat SPS Committee agreed to work to make the provisions of Article 4 more operational (on the basis of W/111)

Developing SPS equivalence guidelines (2) SPS Committee decision on the implementation of Article 4 (G/SPS/19) Oct 2001 March 2002 April 2004 SPS Committee decision – programme for further work (G/SPS/20) Revision 1 (G/SPS/19/Rev. 1) Consider Members’ experiences, 3 Sisters’ work – commence review of decision Clarifications to paragraphs 5, 6 and 7 Update: Reference to OIE and Codex guidelines July 2004 Revision 2 (G/SPS/19/Rev. 2)

Equivalence - Notifications • Member accepting the equivalence of SPS measures of another Member should notify the measure(s) recognized to be equivalent and the products affected by this recognition. • To date, two notifications: Dominican Republic – United States Panama – United States


Thank you! Any questions? Marième Fall Agriculture and Commodities Division marieme. fall@wto. org