The DART Trial Evaluates two strategies for delivering







- Slides: 12

The DART Trial Evaluates two strategies for delivering ART, comparing: • clinical monitoring only with routine laboratory + clinical monitoring • structured treatment interruptions with continuous ART

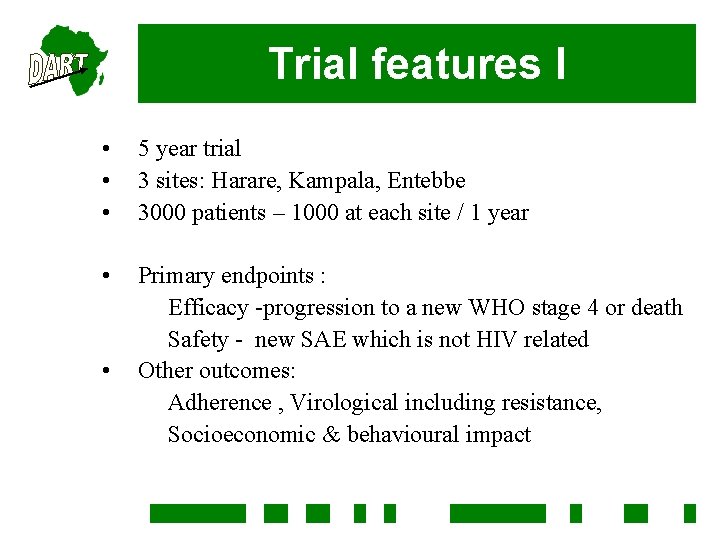
Trial features I • • • 5 year trial 3 sites: Harare, Kampala, Entebbe 3000 patients – 1000 at each site / 1 year • Primary endpoints : Efficacy -progression to a new WHO stage 4 or death Safety - new SAE which is not HIV related Other outcomes: Adherence , Virological including resistance, Socioeconomic & behavioural impact •

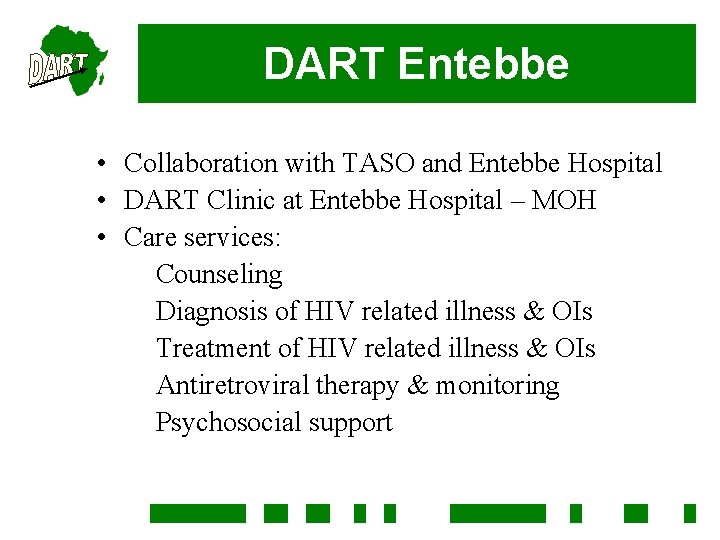
DART Entebbe • Collaboration with TASO and Entebbe Hospital • DART Clinic at Entebbe Hospital – MOH • Care services: Counseling Diagnosis of HIV related illness & OIs Treatment of HIV related illness & OIs Antiretroviral therapy & monitoring Psychosocial support

Funding Sources • Medical Research Council, UK • Rockefeller Foundation, USA • Department for International Development, UK • Rock House Foundation, UK

Drugs Anti-retroviral drugs donated by • Boehringer-Ingelheim - Nevirapine • Gilead - Tenofovir • Glaxo. Smith. Kline - Combivir

Trial Review Process 2 yrs + • • • Risk benefits fine Most attention to end of trial issues Concern about exclusion of pregnant women ? What happens after patient 1000 - community Very little discussion on ability of participants to decline

Trial Approval • UVRI Science & Ethics Committee • Uganda National Council for Science & Technology - granted research clearance • Ethics committees in Zimbabwe & UK • Support letter from MOH

Trial Monitoring • • • Trial Steering Committee International Co-ordinating Group Local Trial Management Committees Analysis & Data Management Committee Data & Safety Monitoring Committee Pharmaceutical companies - adverse events • End Point Review Committee

International Coordinating Group PI’s Site investigators Dr Peter Mugyenyi - Kampala Prof Jimmy Whittworth - Entebbe Prof Ahmed Latif - Zimbabwe Prof James Hakim - Zimbabwe Prof Janet Darbyshire - London Prof Charles Gilks - Geneva Dr Diana Gibb - London Dr Andrew Reid - Zimbabwe Dr Cissy Kityo - Kampala Dr Paula Munderi - Entebbe Plus Dr Dorothy Bray - Consultant Dr Alex Coutinho - TASO Uganda

Other ART Trials in Uganda 1992 JCRC Determining the lowest effective dose of AZT 16 weeks duration 1 yr therapy to participants post trial

Other Trials……………. 2002 Care project ( Pharmaccess, GSK, Roche ) Uganda, Kenya, Senegal, Cote d’Ivoire Response, Side effects, Education & adherence 200 participants total, 50 in each country Will provide ART to participants post trial

Other Trials……………. . 2003 JCRC - NIH Evaluation of short STI strategies ( 7 days on 7 days off / Weekend off ) 171 participants to date Clinical follow up and monitoring provided Patients pay for own ART but save on drug cost ( 50% 7 days ; 30% weekend)
 Identifies, selects, monitors, and evaluates sales channels
Identifies, selects, monitors, and evaluates sales channels Aspire sahs
Aspire sahs Ecological risk assessment definition
Ecological risk assessment definition What divides the nasal cavity
What divides the nasal cavity Xoon expert system
Xoon expert system Esquema dart para extubação
Esquema dart para extubação Animal adaptations in the amazon rainforest
Animal adaptations in the amazon rainforest Dart equivalent
Dart equivalent Vx=vcosθ
Vx=vcosθ Esquema dart para extubação
Esquema dart para extubação Mqtt ventajas y desventajas
Mqtt ventajas y desventajas Dart fedora
Dart fedora Dart: directed automated random testing
Dart: directed automated random testing