Switch to DRVr RAL SPARE Study 118 SPARE

- Slides: 5

Switch to DRV/r + RAL § SPARE Study

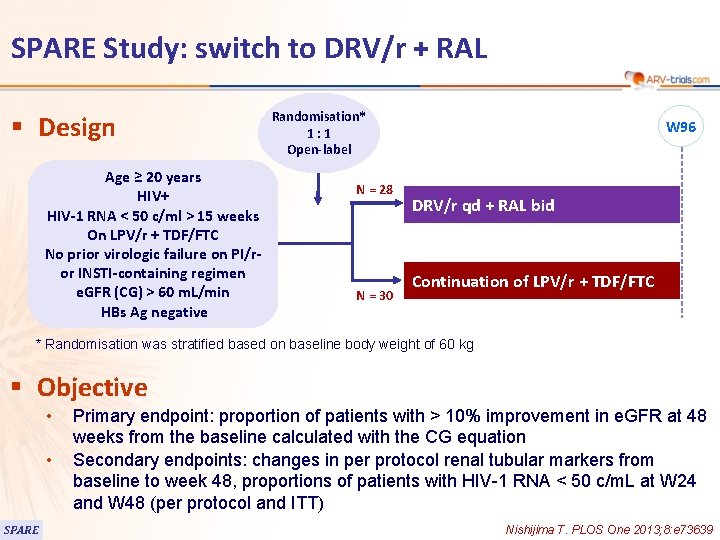
118 SPARE Study: switch to DRV/r + RAL § Design Age ≥ 20 years HIV+ HIV-1 RNA < 50 c/ml > 15 weeks On LPV/r + TDF/FTC No prior virologic failure on PI/ror INSTI-containing regimen e. GFR (CG) > 60 m. L/min HBs Ag negative Randomisation* 1: 1 Open-label N = 28 N = 30 W 96 DRV/r qd + RAL bid Continuation of LPV/r + TDF/FTC * Randomisation was stratified based on baseline body weight of 60 kg § Objective • • SPARE Primary endpoint: proportion of patients with > 10% improvement in e. GFR at 48 weeks from the baseline calculated with the CG equation Secondary endpoints: changes in per protocol renal tubular markers from baseline to week 48, proportions of patients with HIV-1 RNA < 50 c/m. L at W 24 and W 48 (per protocol and ITT) Nishijima T. PLOS One 2013; 8: e 73639

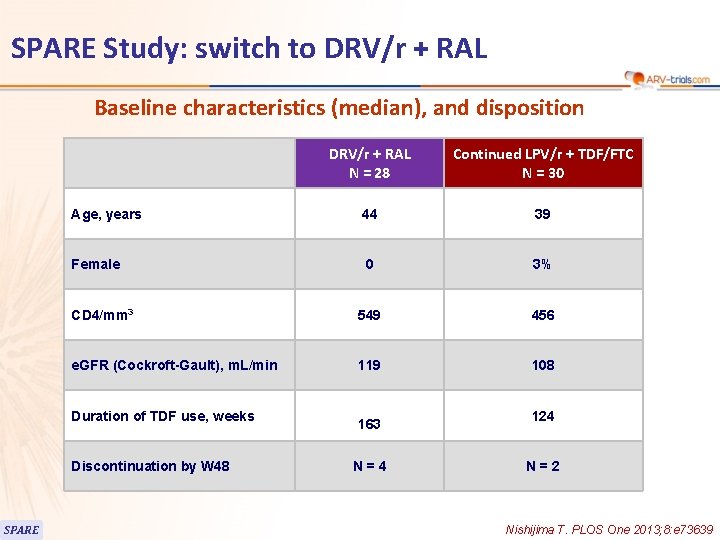
SPARE Study: switch to DRV/r + RAL Baseline characteristics (median), and disposition DRV/r + RAL N = 28 Continued LPV/r + TDF/FTC N = 30 Age, years 44 39 Female 0 3% CD 4/mm 3 549 456 e. GFR (Cockroft-Gault), m. L/min 119 108 Duration of TDF use, weeks Discontinuation by W 48 SPARE 163 N=4 124 N=2 Nishijima T. PLOS One 2013; 8: e 73639

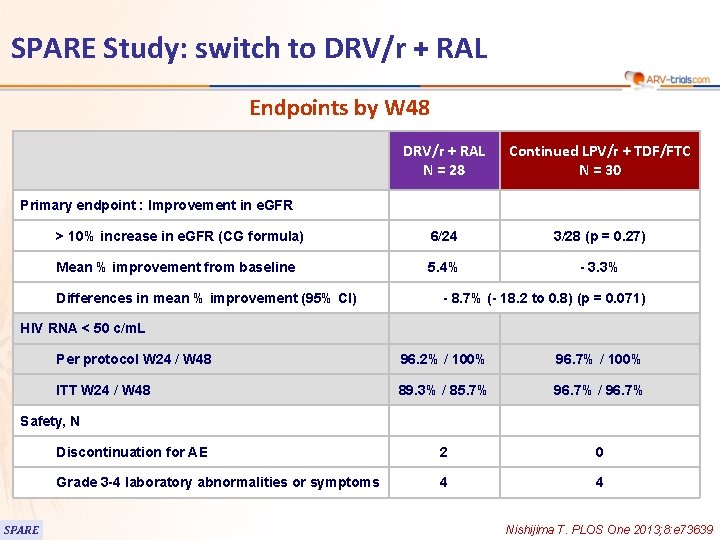
SPARE Study: switch to DRV/r + RAL Endpoints by W 48 DRV/r + RAL N = 28 Continued LPV/r + TDF/FTC N = 30 > 10% increase in e. GFR (CG formula) 6/24 3/28 (p = 0. 27) Mean % improvement from baseline 5. 4% - 3. 3% Primary endpoint : Improvement in e. GFR Differences in mean % improvement (95% CI) - 8. 7% (- 18. 2 to 0. 8) (p = 0. 071) HIV RNA < 50 c/m. L Per protocol W 24 / W 48 96. 2% / 100% 96. 7% / 100% ITT W 24 / W 48 89. 3% / 85. 7% 96. 7% / 96. 7% Discontinuation for AE 2 0 Grade 3 -4 laboratory abnormalities or symptoms 4 4 Safety, N SPARE Nishijima T. PLOS One 2013; 8: e 73639

SPARE Study: switch to DRV/r + RAL § Conclusion – Switching LPV/r + TDF/FTC to RAL+ DRV/r did not significantly increase the proportion of patients who showed >10% improvement in renal function among those with relatively preserved e. GFR. However, the switch improved urinary β 2 microglobulin, suggesting that discontinuation of TDF might be beneficial in the long-term – RAL +DRV/r showed favorable viral efficacy in patients with suppressed viral load – Limitations • Small sample size • Adverse events self-reported, open-label unblinded design SPARE Nishijima T. PLOS One 2013; 8: e 73639