Switch to ATV rcontaining regimen SWAN SLOAT SWAN

- Slides: 8

Switch to ATV + r-containing regimen - SWAN - SLOAT

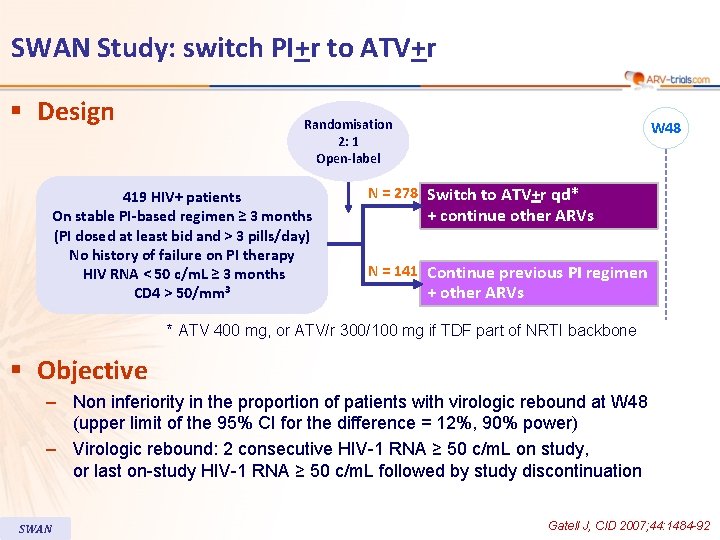
SWAN Study: switch PI+r to ATV+r § Design Randomisation 2: 1 Open-label 419 HIV+ patients On stable PI-based regimen ≥ 3 months (PI dosed at least bid and > 3 pills/day) No history of failure on PI therapy HIV RNA < 50 c/m. L ≥ 3 months CD 4 > 50/mm 3 W 48 N = 278 Switch to ATV+r qd* + continue other ARVs N = 141 Continue previous PI regimen + other ARVs * ATV 400 mg, or ATV/r 300/100 mg if TDF part of NRTI backbone § Objective – Non inferiority in the proportion of patients with virologic rebound at W 48 (upper limit of the 95% CI for the difference = 12%, 90% power) – Virologic rebound: 2 consecutive HIV-1 RNA ≥ 50 c/m. L on study, or last on-study HIV-1 RNA ≥ 50 c/m. L followed by study discontinuation SWAN Gatell J, CID 2007; 44: 1484 -92

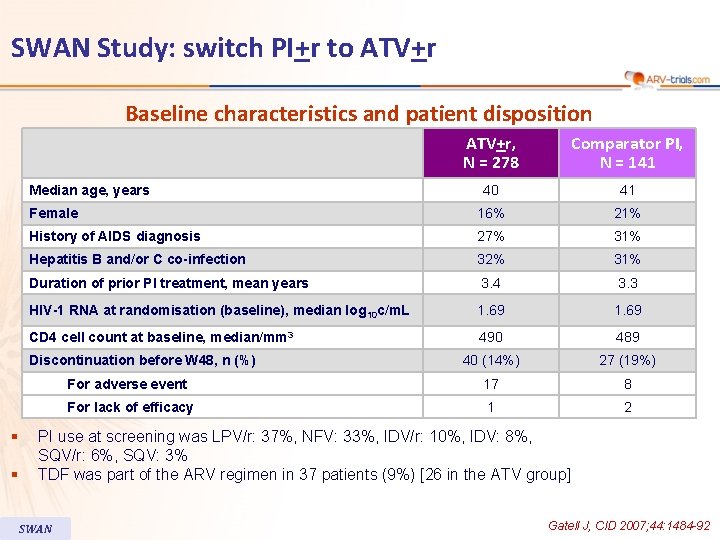
SWAN Study: switch PI+r to ATV+r Baseline characteristics and patient disposition ATV+r, N = 278 Comparator PI, N = 141 40 41 Female 16% 21% History of AIDS diagnosis 27% 31% Hepatitis B and/or C co-infection 32% 31% Duration of prior PI treatment, mean years 3. 4 3. 3 HIV-1 RNA at randomisation (baseline), median log 10 c/m. L 1. 69 CD 4 cell count at baseline, median/mm 3 490 489 40 (14%) 27 (19%) For adverse event 17 8 For lack of efficacy 1 2 Median age, years Discontinuation before W 48, n (%) § § PI use at screening was LPV/r: 37%, NFV: 33%, IDV/r: 10%, IDV: 8%, SQV/r: 6%, SQV: 3% TDF was part of the ARV regimen in 37 patients (9%) [26 in the ATV group] SWAN Gatell J, CID 2007; 44: 1484 -92

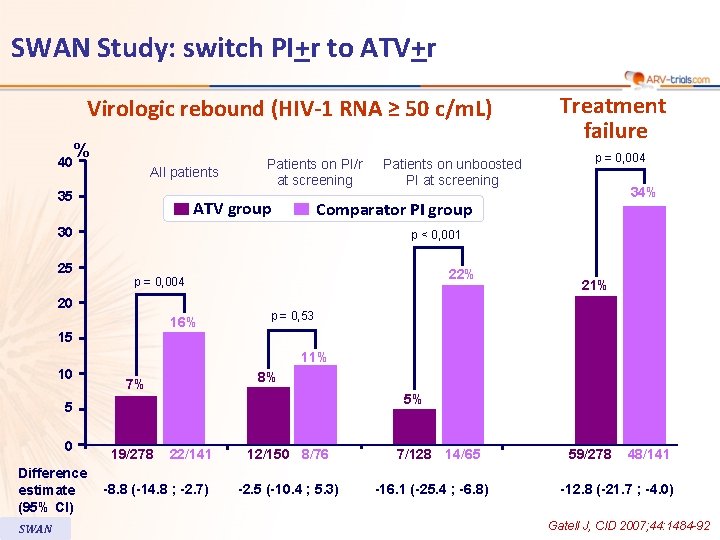
SWAN Study: switch PI+r to ATV+r Virologic rebound (HIV-1 RNA ≥ 50 c/m. L) 40 % All patients 35 Patients on PI/r at screening ATV group p = 0, 004 34% Comparator PI group 30 25 Patients on unboosted PI at screening Treatment failure p < 0, 001 22% p = 0, 004 20 16% 21% p = 0, 53 15 11% 10 8% 7% 5% 5 0 Difference estimate (95% CI) SWAN 19/278 22/141 -8. 8 (-14. 8 ; -2. 7) 12/150 8/76 -2. 5 (-10. 4 ; 5. 3) 7/128 14/65 -16. 1 (-25. 4 ; -6. 8) 59/278 48/141 -12. 8 (-21. 7 ; -4. 0) Gatell J, CID 2007; 44: 1484 -92

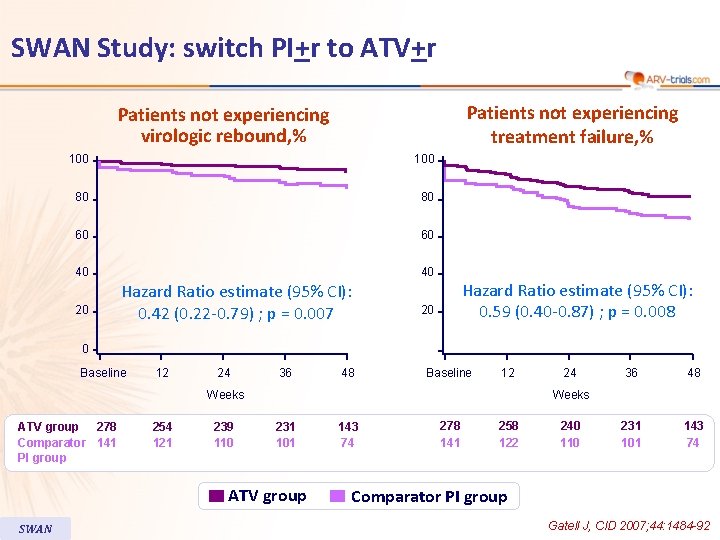
SWAN Study: switch PI+r to ATV+r Patients not experiencing treatment failure, % Patients not experiencing virologic rebound, % 100 80 80 60 60 40 40 20 Hazard Ratio estimate (95% CI): 0. 42 (0. 22 -0. 79) ; p = 0. 007 Hazard Ratio estimate (95% CI): 0. 59 (0. 40 -0. 87) ; p = 0. 008 20 0 Baseline 12 24 36 48 Baseline 12 Weeks ATV group 278 Comparator 141 PI group 254 121 239 110 36 48 231 101 143 74 Weeks 231 101 ATV group SWAN 24 143 74 278 141 258 122 240 110 Comparator PI group Gatell J, CID 2007; 44: 1484 -92

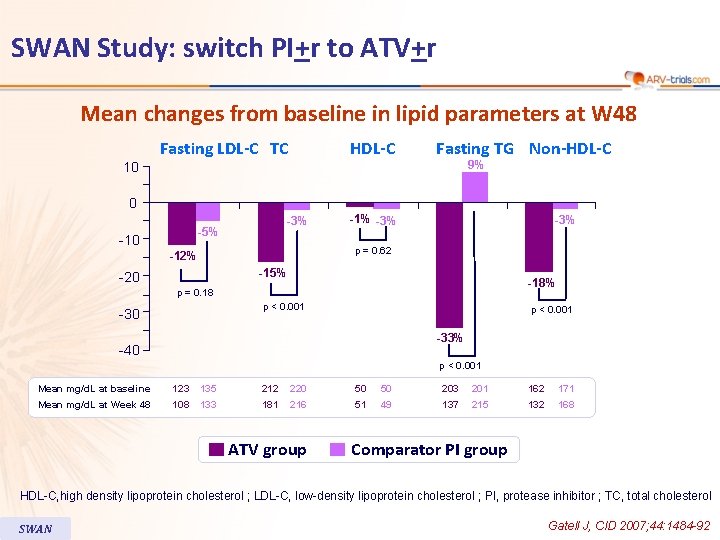
SWAN Study: switch PI+r to ATV+r Mean changes from baseline in lipid parameters at W 48 10 Fasting LDL-C TC HDL-C Fasting TG Non-HDL-C 9% 0 -3% -5% -10 -1% -3% p = 0. 62 -12% -15% -20 -18% p = 0. 18 p < 0. 001 -30 p < 0. 001 -33% -40 p < 0. 001 Mean mg/d. L at baseline 123 135 212 220 50 50 203 201 162 171 Mean mg/d. L at Week 48 108 133 181 216 51 49 137 215 132 168 ATV group Comparator PI group HDL-C, high density lipoprotein cholesterol ; LDL-C, low-density lipoprotein cholesterol ; PI, protease inhibitor ; TC, total cholesterol SWAN Gatell J, CID 2007; 44: 1484 -92

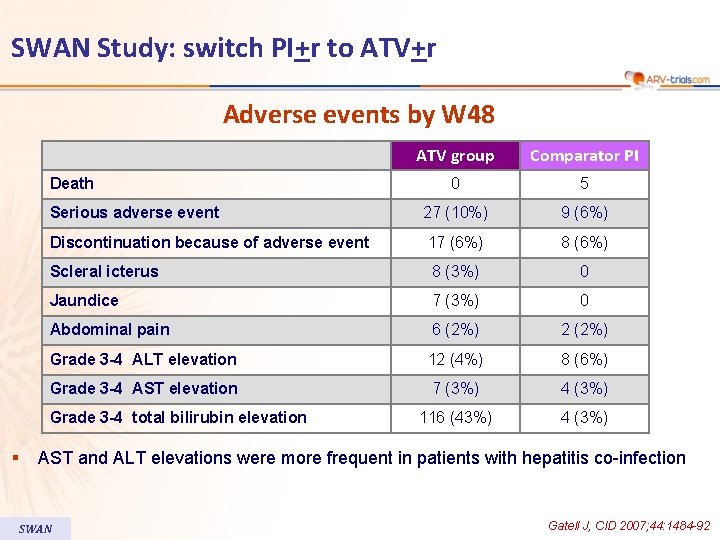
SWAN Study: switch PI+r to ATV+r Adverse events by W 48 ATV group Comparator PI 0 5 Serious adverse event 27 (10%) 9 (6%) Discontinuation because of adverse event 17 (6%) 8 (6%) Scleral icterus 8 (3%) 0 Jaundice 7 (3%) 0 Abdominal pain 6 (2%) 2 (2%) Grade 3 -4 ALT elevation 12 (4%) 8 (6%) Grade 3 -4 AST elevation 7 (3%) 4 (3%) 116 (43%) 4 (3%) Death Grade 3 -4 total bilirubin elevation § AST and ALT elevations were more frequent in patients with hepatitis co-infection SWAN Gatell J, CID 2007; 44: 1484 -92

SWAN Study: switch PI+r to ATV+r § Conclusions – Switching to a simplified PI-based regimen containing ATV provided better maintenance of virologic suppression with lower rates of virologic rebound and treatment failure than those observed with continued, unmodified therapy – Safety and tolerability were similar in both groups • But lipid parameters improved in the ATV group • Hyperbilirubinemia was frequent on ATV SWAN Gatell J, CID 2007; 44: 1484 -92