Solving Example 7 6 p 273 th Jespersen



- Slides: 4

Solving Example 7. 6 p. 273 th Jespersen 6 Edition 1

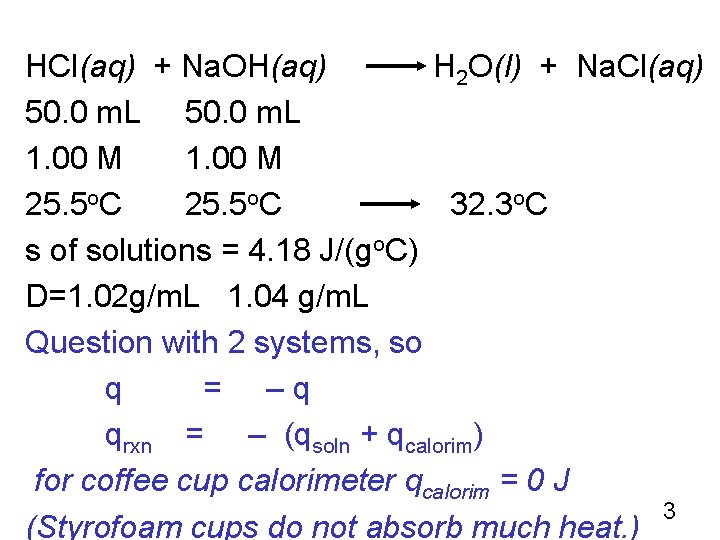
Example 7. 6 p. 273 HCl(aq) + Na. OH(aq) H 2 O(l) + Na. Cl(aq) A student placed 50. 0 m. L of 1. 00 M HCl at 25. 5 o. C in a coffee cup calorimeter. To this was added 50. 0 m. L of 1. 00 M Na. OH soln also at 25. 5 o. C. The mixture was stirred and temp quickly increased to a max of 32. 2 o. C. What is ΔH in k. J per mole of HCl? Because the solns are relatively dilute, we can assume that their specific heats are close to that of water, 4. 18 J g-1 o. C-1. The density of 1. 00 M HCl is 1. 02 g/m. L and that of 1. 00 M Na. OH is 1. 04 g/m. L. (We will neglect the heat lost to the Styrofoam itself, to thermometer or to the surrounding air. ) 2

HCl(aq) + Na. OH(aq) H 2 O(l) + Na. Cl(aq) 50. 0 m. L 1. 00 M 25. 5 o. C 32. 3 o. C s of solutions = 4. 18 J/(go. C) D=1. 02 g/m. L 1. 04 g/m. L Question with 2 systems, so q = –q qrxn = – (qsoln + qcalorim) for coffee cup calorimeter qcalorim = 0 J 3 (Styrofoam cups do not absorb much heat. )

qrxn = – (qsoln + qcalorim) qrxn = – (qsoln + 0) qsoln = s m T where s = 4. 18 J/(g. o. C) m= mass of soln = ? T = (32. 2 -25. 5)o. C = 6. 7 o. C Mass of soln = mass. HCl + mass. Na. OH m of HCl = 50. 0 m. L HCl x 1. 02 g/m. L = 51. 0 g m of Na. OH = 50. 0 m. L Na. OH x 1. 04 g/m. L = 52. 0 g m of soln = 51. 0 g + 52. 0 g = 103. 0 g qsoln = s m T = (4. 18 J/(g. o. C))(103. 0 g)(6. 7 o. C) = 2, 884. 6 J = 2. 88 k. J Ques asked for H in k. J per mole HCl. Is this for 1 mol HCl? NO! Our ans of 2. 88 k. J is for how many moles? x mol HCl = 0. 0500 L x 1. 00 mol HCl/L = 0. 0500 mol HCl Is that H ? . . . Not quite. . . q rxn = - q soln = - 58 k. J/mol HCl Ans. H = - 58 k. J/mol HCl For an open vessel, q = H 4