Skin SSG Research 18 th June 2015 Dr








- Slides: 11

Skin SSG – Research 18 th June 2015 Dr Toby Talbot, Consultant Oncologist Wendy Cook, Research Delivery Manager Delivering clinical research to make patients, and the NHS, better

South West Peninsula CRN Hospital Size Yeovil District Hospital NHS Foundation Trust Small Acute Taunton and Somerset NHS Foundation Trust Medium Acute Northern Devon Healthcare NHS Trust Small Acute Royal Devon and Exeter NHS Foundation Trust Large Acute South Devon Healthcare NHS Foundation Trust Medium Acute Plymouth Hospitals NHS Trust Large Acute Royal Cornwall Hospitals NHS Trust Large Acute

Division 1 - Cancer - Contacts • Duncan Wheatley, Clinical Specialty Lead duncan. wheatley@rcht. cornwall. nhs. uk 01872 258312 • Toby Talbot, Sub specialty lead • toby. talbot@nhs. net 01872 258314 • Wendy Cook, Research delivery manager wcook 1@nhs. net 01392 406995 • Ann Courtman, Administrator ann. courtman@nhs. net 01392 406966 3

NIHR Cancer Research Objectives • Increase the opportunities for cancer patients to take part in research studies, regardless of where they live • Develop an action plan to increase access in each subspecialty (e. g by opening studies, increasing awareness and forming referral pathways for access to research) 4

NIHR Cancer Research Objectives • Increase the number of cancer patients participating in studies, to support the national target of 20% cancer incidence – SW Peninsula Cancer Incidence 12, 580 – 20% = 2, 516 pts – Current performance – 1535 pts 12. 2% • Increase the number of cancer patients participating in interventional trials, to support the national target of 7. 5% cancer incidence – 7. 5% = 944 pts – Current performance – 714 pts 5. 7% This is measured on 12 months data from ODP 5

What we need to achieve in each subspecialty group • 13 named cancer sub specialty leads with a defined portfolio of studies • Highlight, promote studies • Promote referral pathways throughout the network • Research Subspecialty Lead – Participate in national meetings to collaborate with other sub-speciality leads, identify studies to bring to the region – Connection with CSG’s, feedback re sub specialty portfolio and availability of trials for all. 6

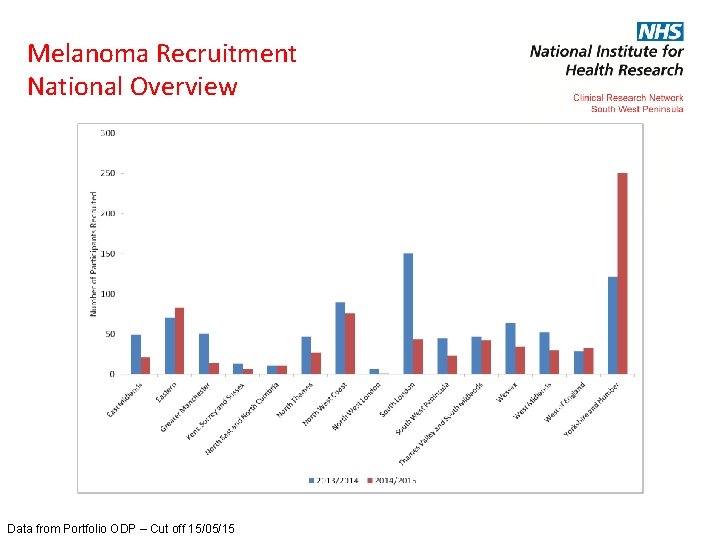
Melanoma Recruitment National Overview Data from Portfolio ODP – Cut off 15/05/15

Melanoma Recruitment National Overview CRN 2013/2014/2015 East Midlands 49 21 Eastern 70 83 Greater Manchester 51 14 Kent, Surrey and Sussex 13 7 North East and North Cumbria 11 11 North Thames 47 27 North West Coast 90 76 North West London 7 1 South London 151 44 South West Peninsula 45 23 Thames Valley and South Midlands 47 42 Wessex 64 34 West Midlands 52 30 West of England 28 33 Yorkshire and Humber 122 250 Data from Portfolio ODP – Cut off 15/05/15

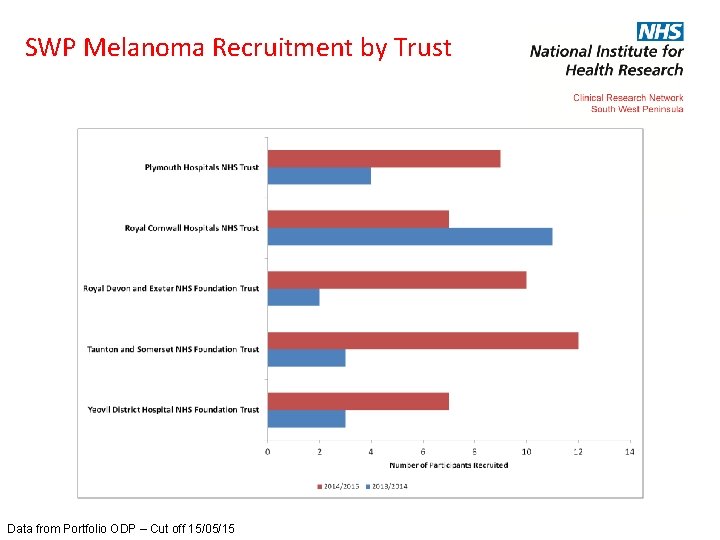
SWP Melanoma Recruitment by Trust Data from Portfolio ODP – Cut off 15/05/15

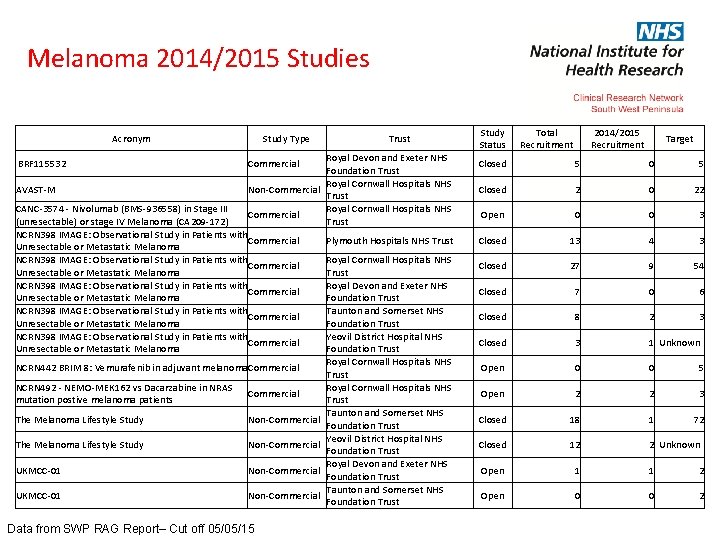
Melanoma 2014/2015 Studies Acronym BRF 115532 AVAST-M Study Type Trust Royal Devon and Exeter NHS Foundation Trust Royal Cornwall Hospitals NHS Non-Commercial Trust Royal Cornwall Hospitals NHS Commercial Trust Commercial CANC-3574 - Nivolumab (BMS-936558) in Stage III (unresectable) or stage IV Melanoma (CA 209 -172) NCRN 398 IMAGE: Observational Study in Patients with Commercial Unresectable or Metastatic Melanoma NCRN 398 IMAGE: Observational Study in Patients with Commercial Unresectable or Metastatic Melanoma NCRN 442 BRIM 8: Vemurafenib in adjuvant melanoma. Commercial NCRN 492 - NEMO-MEK 162 vs Dacarzabine in NRAS mutation postive melanoma patients Commercial The Melanoma Lifestyle Study Non-Commercial UKMCC-01 Non-Commercial Data from SWP RAG Report– Cut off 05/05/15 Plymouth Hospitals NHS Trust Royal Cornwall Hospitals NHS Trust Royal Devon and Exeter NHS Foundation Trust Taunton and Somerset NHS Foundation Trust Yeovil District Hospital NHS Foundation Trust Royal Cornwall Hospitals NHS Trust Taunton and Somerset NHS Foundation Trust Yeovil District Hospital NHS Foundation Trust Royal Devon and Exeter NHS Foundation Trust Taunton and Somerset NHS Foundation Trust Study Status Total Recruitment 2014/2015 Recruitment Target Closed 5 0 5 Closed 2 0 22 Open 0 0 3 Closed 13 4 3 Closed 27 9 54 Closed 7 0 6 Closed 8 2 3 Closed 3 1 Unknown Open 0 0 5 Open 2 2 3 Closed 18 1 72 Closed 12 2 Unknown Open 1 1 2 Open 0 0 2

Portfolio maps link http: //csg. ncri. org. uk/portfolio-maps/ • Moving forward the Network aim is to map sites open to studies on the portfolio maps • This is large piece of work and will take some time 11





















