REGULATORY ISSUES IN HIV CURE RESEARCH HIV Cure








- Slides: 18

REGULATORY ISSUES IN HIV CURE RESEARCH HIV Cure Research Training Curriculum Regulatory Issues Module by: Damon Deming, Ph. D. FDA Division of Antiviral Products Nov 2014 The HIV CURE training curriculum is a collaborative project aimed at making HIV cure research science accessible to the community and the HIV research field. The opinions expressed in this module are those of the author and may not represent official FDA policy.

OUTLINE 1 Overview of the Regulatory Process 2 Regulatory Decision Making 3 Regulatory Issues in HIV Cure Research 4 Patient Role in the Regulatory Process 2

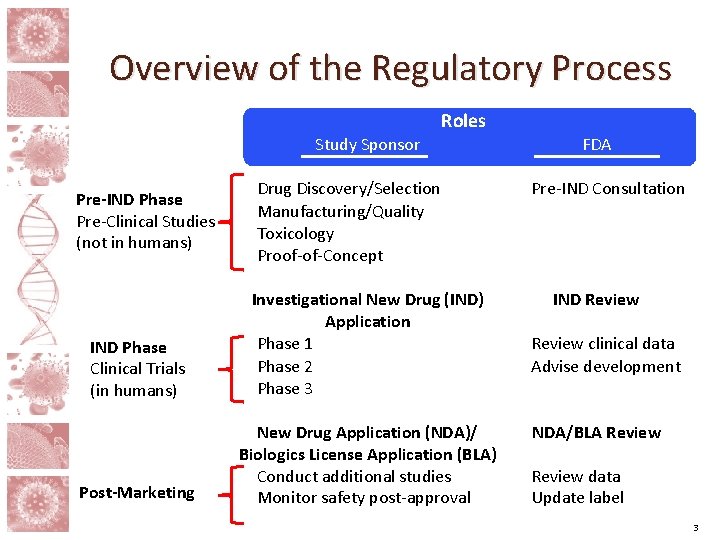
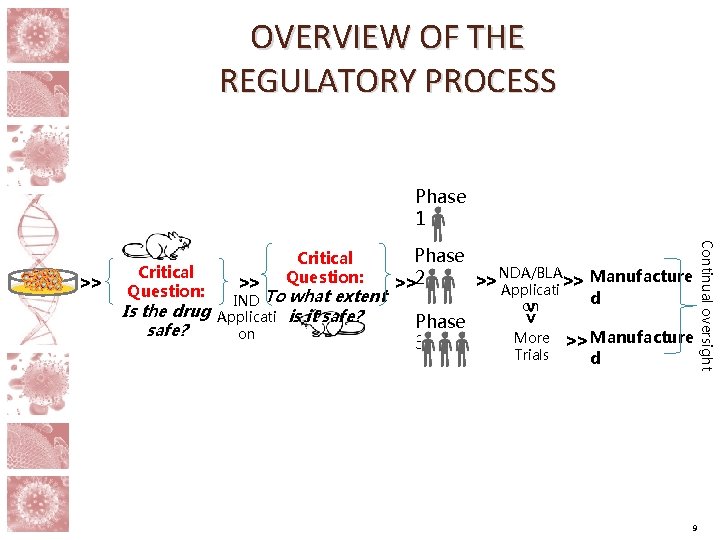
Overview of the Regulatory Process Roles Study Sponsor Pre-IND Phase Pre-Clinical Studies (not in humans) Drug Discovery/Selection Manufacturing/Quality Toxicology Proof-of-Concept IND Phase Clinical Trials (in humans) Investigational New Drug (IND) Application Phase 1 Phase 2 Phase 3 Post-Marketing New Drug Application (NDA)/ Biologics License Application (BLA) Conduct additional studies Monitor safety post-approval FDA Pre-IND Consultation IND Review clinical data Advise development NDA/BLA Review data Update label 3

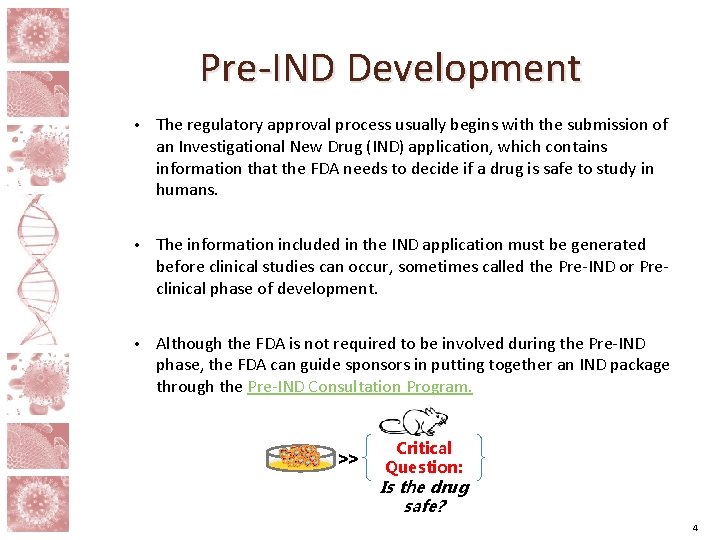
Pre-IND Development • The regulatory approval process usually begins with the submission of an Investigational New Drug (IND) application, which contains information that the FDA needs to decide if a drug is safe to study in humans. • The information included in the IND application must be generated before clinical studies can occur, sometimes called the Pre-IND or Preclinical phase of development. • Although the FDA is not required to be involved during the Pre-IND phase, the FDA can guide sponsors in putting together an IND package through the Pre-IND Consultation Program. >> Critical Question: Is the drug safe? 4

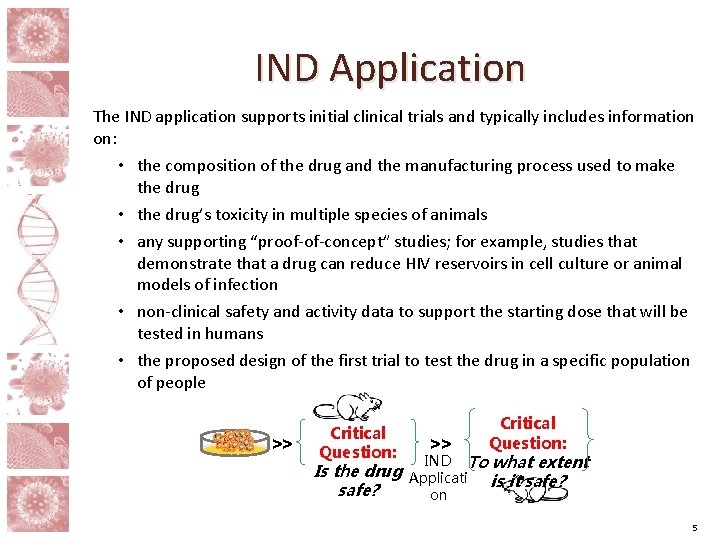
IND Application The IND application supports initial clinical trials and typically includes information on: • the composition of the drug and the manufacturing process used to make the drug • the drug’s toxicity in multiple species of animals • any supporting “proof-of-concept” studies; for example, studies that demonstrate that a drug can reduce HIV reservoirs in cell culture or animal models of infection • non-clinical safety and activity data to support the starting dose that will be tested in humans • the proposed design of the first trial to test the drug in a specific population of people >> Critical Question: IND To what extent Is the drug Applicati is it safe? on 5

IND Clinical Development A series of progressively larger clinical trials are conducted as more information is learned about the safety and potential efficacy of the drug. The trials are typically separated into three phases of development: Phase I: Phase III: THESE TRIALS include several small trials designed to learn how the drug is broken down by the body and to begin studying the drug’s safety in humans. These larger trials look for evidence that the drug is acting as expected and compare different ways of giving the drug in order to find a safe and potentially effective dosing strategy. These MUCH large. R trials are intended to verify that the drug is safe and prove that the drug is effective. Phase 1 Critical Question: To what extent is it safe? Phase >> >>2 Phase 3 6

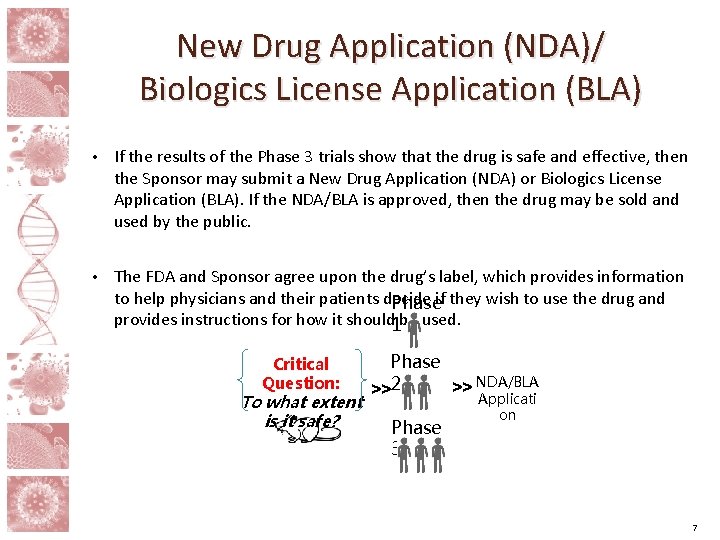
New Drug Application (NDA)/ Biologics License Application (BLA) • If the results of the Phase 3 trials show that the drug is safe and effective, then the Sponsor may submit a New Drug Application (NDA) or Biologics License Application (BLA). If the NDA/BLA is approved, then the drug may be sold and used by the public. • The FDA and Sponsor agree upon the drug’s label, which provides information to help physicians and their patients decide Phaseif they wish to use the drug and provides instructions for how it should 1 be used. Critical Question: To what extent is it safe? Phase >> NDA/BLA >>2 Applicati Phase 3 on 7

Post-Marketing • The FDA may require that additional non-clinical studies or clinical trials be completed after the NDA/BLA is approved. For example, an additional clinical study might be conducted to provide information for a group of people who might benefit from the drug but who were not included in previous trials. • The FDA continues to receive and review long-term safety information for approved drugs. Identification of new safety issues could require changes to the drug’s label or, in extreme cases, withdrawal of the drug from the market. Phase 1 >> Phase 3 on More Trials d >> Manufacture d Continual oversight Phase NDA/BLA 2 >> Applicati >> Manufacture 8

OVERVIEW OF THE REGULATORY PROCESS Phase 1 Critical Question: IND To what extent Applicati is it safe? on Phase 3 on >> Is the drug safe? >> Phase NDA/BLA >> Applicati >> Manufacture >>2 More Trials d >> Manufacture d 9 Continual oversight >> Critical Question:

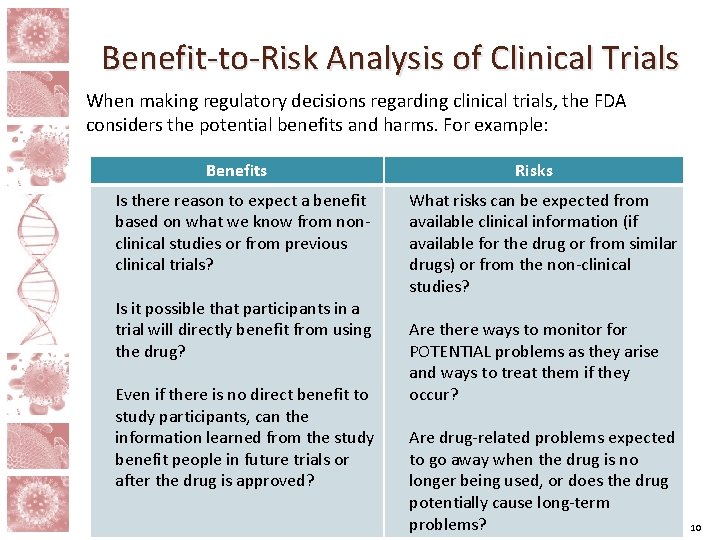
Benefit-to-Risk Analysis of Clinical Trials When making regulatory decisions regarding clinical trials, the FDA considers the potential benefits and harms. For example: Benefits Is there reason to expect a benefit based on what we know from nonclinical studies or from previous clinical trials? Is it possible that participants in a trial will directly benefit from using the drug? Even if there is no direct benefit to study participants, can the information learned from the study benefit people in future trials or after the drug is approved? Risks What risks can be expected from available clinical information (if available for the drug or from similar drugs) or from the non-clinical studies? Are there ways to monitor for POTENTIAL problems as they arise and ways to treat them if they occur? Are drug-related problems expected to go away when the drug is no longer being used, or does the drug potentially cause long-term problems? 10

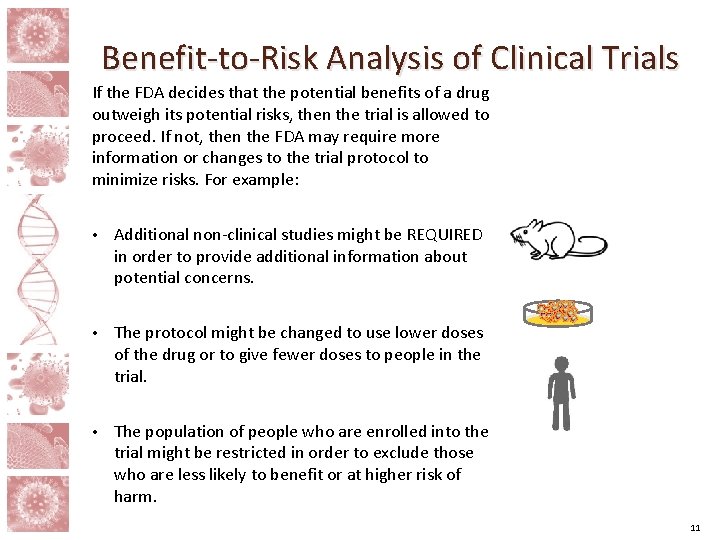
Benefit-to-Risk Analysis of Clinical Trials If the FDA decides that the potential benefits of a drug outweigh its potential risks, then the trial is allowed to proceed. If not, then the FDA may require more information or changes to the trial protocol to minimize risks. For example: • Additional non-clinical studies might be REQUIRED in order to provide additional information about potential concerns. • The protocol might be changed to use lower doses of the drug or to give fewer doses to people in the trial. • The population of people who are enrolled into the trial might be restricted in order to exclude those who are less likely to benefit or at higher risk of harm. 11

Regulatory Issues in HIV Cure Trials Like all clinical trials, HIV cure-related trials are evaluated by considering potential benefits and risks. Potential benefits: • Initial trials are necessary proof-of-concept studies intended to evaluate particular aspects of biological activity that might be a useful step towards developing a cure. For example, a drug might be studied for its ability to activate latently-infected cells in the body. • Although there is little prospect of a direct benefit to the people who participate in early phase trials, the results from these trials might be useful for designing later studies and advancing the development of a safe and effective cure. 12

Regulatory Issues in HIV Cure Trials The FDA also considers potential risks of early HIV cure-related trials: • These studies are not expected to result in a cure, and the lack of direct benefit to trial participants requires that those people are not exposed to unreasonable risk. • The potential of the drug or trial procedures to compromise the effectiveness of antiretroviral drug treatment IS CONSIDERED. • Safety in new populations: Acceptable risk levels may be different in populations for which a drug has been PROVEN TO BE SAFE AND EFFECTIVE (e. g. , cancer) versus an investigational population (e. g. , HIV-infected) for which the drug has no proven benefit AND MAY BE HARMFUL. 13

Other HIV Cure-Related Issues • Regulatory issues are often related to scientific issues ‒ WE need for biomarkers that can be used to determine treatment effects; for example, changes in the number of latently-infected cells. ‒ We need for new assays that may be sensitive enough to detect clinically significant changes in those biomarkers. ‒ We need NEW assays may also need to be approved as a companion diagnostic for HIV cure drugs; that is, physicians may need access to those assays in order to properly administer an HIV cure therapy. • Antiretroviral treatment interruption (ATI) ‒ Cure trials will ultimately require an interruption of antiretroviral drugs in order to determine if study subjects have been cured. ‒ Ideally, there will be a useful biomarker that can be used to predict success before A TREATMENT interruption in order to limit the risks associated with treatment interruptions, including periods of uncontrolled virus replication and minimize the risks associated with that replication; (e. g. , selecting resistant virus AND increased risk of transmission). 14

Other HIV Cure-Related Issues • Development for special populations (e. g. , pediatrics) ‒ THE FDA will first require the generation of substantial safety and efficacy data in adults. • Trial durations may be very long ‒ It is not known how long virus will need to remain undetected once off of antiretroviral treatment before a person can be considered cured because virus may take several months to rebound. ‒ It is also unclear how long people should be followed for safety in order to detect slowly developing adverse events that might be related to the HIV cure treatment; e. g. , cancer risks. • Combination products ‒ ‒ An HIV cure may involve a combination OF products; (e. g. , drug/biologic. ) THE Contribution of each component to efficacy must be demonstrated. THE Components may have distinct regulatory requirements. Review by different FDA Centers MAY BE REQUIRED. 15

Other HIV Cure-Related Issues • It is not currently known what an “HIV cure” will look like ‒ Ideally, a cure will result in removal of all virus from the body. ‒ However, it is possible that a permanent or temporary reduction in viral load without antiretroviral drugs might be useful even if a complete cure is not found. § Approval of such therapies would likely depend on several factors, such as: ‒ the safety of therapy ‒ the target population of HIV-infected people that therapy is intended to TREAT ‒ the magnitude and duration of viral suppression, and ‒ the potential for re-treatment. 16

Patient Role in the Regulatory Process • Volunteers should understand the potential benefits and risks of participating in a trial. • Patients and trial volunteers can help regulators, sponsors, and clinical trial investigators define the acceptable levels of risk for clinical trials. • The FDA greatly values the perspective and input of patients and potential clinical trial participants. The community is strongly encouraged to provide feedback to regulators: ‒ Through COMMUNITY ADVISORY BOARDS and trial sponsors ‒ During public meetings ‒ During NDA/BLA advisory committee meetings 17

CONCLUSIONS • The development of an HIV cure NEEDS TO work within the framework of the regulatory process. • Regulatory decisions are based on benefit-to-risk analyses. • Regulatory uncertainty regarding HIV cure research is largely related to scientific uncertainty. • The regulatory path to approval is expected to evolve with advances in the science DISCOVERED THROUGH potential HIV cure strategies. • There is an important role for patients in the HIV cure development process. 18
 Arv copenhagen
Arv copenhagen Legal regulatory and political issues
Legal regulatory and political issues Touching spirit bear questions
Touching spirit bear questions Marié cure
Marié cure Define curing of concrete
Define curing of concrete Cure: an efficient clustering algorithm for large databases
Cure: an efficient clustering algorithm for large databases The cure that time forgot
The cure that time forgot Nuclei di cure primarie
Nuclei di cure primarie Cold cure acrylic resin composition
Cold cure acrylic resin composition External fragmentation
External fragmentation The cure that time forgot
The cure that time forgot Log80 ausl romagna
Log80 ausl romagna Dr gary kroukamp
Dr gary kroukamp Otr tire curing press
Otr tire curing press Dipartimento cure primarie bologna
Dipartimento cure primarie bologna Kitchenthusiast contributor
Kitchenthusiast contributor Cure for cipa
Cure for cipa Cure programmer
Cure programmer Cross linking in denture base resin is contributed by
Cross linking in denture base resin is contributed by