ETHICAL CONSIDERATIONS OF HIV CURE RESEARCH HIV Cure






















- Slides: 38

ETHICAL CONSIDERATIONS OF HIV CURE RESEARCH HIV Cure Research Training Curriculum Ethics of HIV Cure Research Module by: Dr. Stuart Rennie, Ph. D, MA University of North Carolina, Chapel Hill Jessica Handibode, MBE, AVAC and Karine Dubé, CARE August 2015 The HIV CURE research training curriculum is a collaborative project aimed at making HIV cure research science accessible to the community and the HIV research field.

Session objectives • Understanding (research) ethics • Distinguishing ethics of HIV prevention, treatment and cure research • Ethical highlight: research involving treatment interruption • Challenges unique to different research modalities

Understanding ethics • What is morality? • Practices of considering activities as right/wrong (etc. ) according to traditional values • What is ethics? • Identifying, analyzing and resolving conflicts of value • Research with human participants involves values and they can conflict

Values in research ethics • Scientific validity • Is the research designed well enough to answer the research question? • Social value • Is the research likely to foster scientific progress and provide an important benefit to society? • Scalability • Can the end products of the research be implemented on a large scale to those who need it? • Fair participant selection • Are benefits and burdens equitably distributed between selected research participants and potential research beneficiaries? Adapted from Lo and Grady, Curr Opin HIV AIDS 2013

Values in research ethics • Favorable risk/benefit ratio • Are risks to participants minimized and acceptable in view of personal or social benefits? • • Informed consent, confidentiality, privacy • Are participants empowered to participate on the basis of adequate understanding? Is what they disclose during research involvement protected? • Community engagement • Are communities impacted by the research meaningfully involved at all stages of the research? • Independent review • Will the research be subject to scientific and ethical evaluation by legitimate third parties? Adapted from Lo and Grady, Curr Opin HIV AIDS 2013

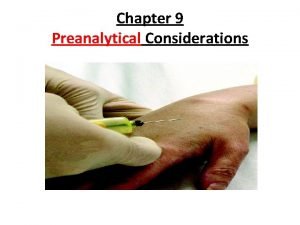
Ethics in HIV treatment, prevention and cure research Research type Participant status Research goal Selected ethical issues HIV treatment HIV positive Effective suppression of virus, boosting immune system Risk of drug sideeffects; adherence problems HIV prevention HIV negative Effective methods of preventing HIV acquisition Seroconversion of participants during trial; behavioral disinhibition HIV cure HIV positive Interventions to produce permanently suppress or eradicate HIV Risk of intervention side-effects; existence of known effective treatment

Selected ethical challenges • Social value • Scalability • Informed consent • Favorable risk/benefit ratio • Whose risk? Issue of partner involvement • Studies involving analytic treatment interruption (ATI)

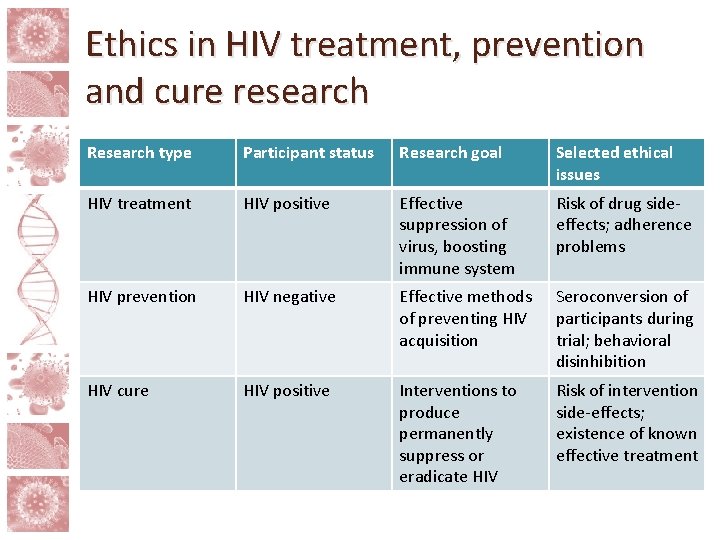
What is the potential social value of an HIV cure? For patients/participants • Suspension of lifelong c. ART and its short and long term side-effects • Potential for reduced stigma For public health • To impact/stop HIV epidemic by lowering transmission • To reduce high-cost expenditures related to HIV care and treatment

Tensions between domains HIV prevention and treatment efforts: and now cure? • Considerations: • • • Burdens and limitation of HIV treatment Cure ≠ the ‘end of AIDS’ (cf. TB) Cure research where treatment access is partial? Research on acutes, global burden = chronic Reasons to pursue HIV cure vs. reasons to improve ART or prevention/prophylactic methods

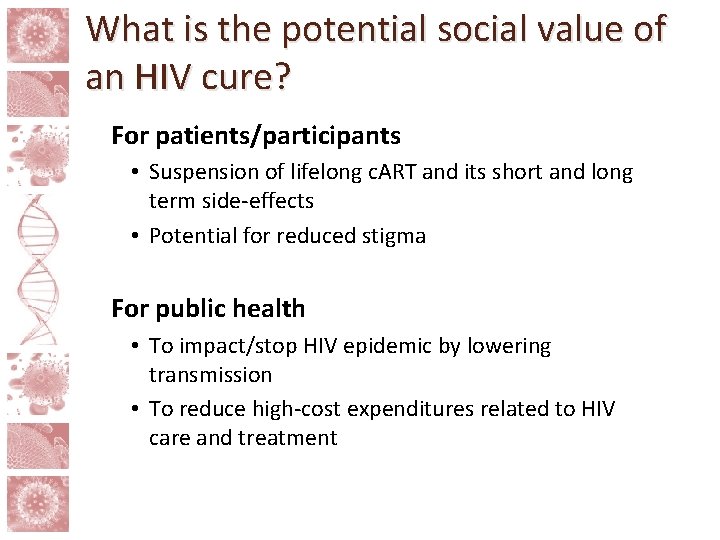
Scalability In terms of scalability, not all current HIV cure approaches are created equal Approach Stage Safety Scalability ‘Shock and kill’ Clinical/preclinical Medium Gene manipulation Clinical Medium (long term effects unknown) Very low Stem cell transplants Clinical Very low Immune based therapies Clinical High Medium Adapted from Shytaj IL and Savarino (2013)

Language of cure Study, trial or experiment? • Control, remission or cure? • Patient, participant or subject? •

Informed Consent • People should be empowered to freely decide whether to participate in cure research on the basis of adequate understanding • Therapeutic misconception: false belief that the aim of research is to produce a personal health benefit for research participants • Curative misconception: false belief that the aim of HIV cure research is to provide a cure for research participants

Informed consent • Even without misconceptions, cure research is scientifically and socially complex = need for mutual literacy • Scientific literacy: initiatives to translate research into accessible lay terms • Community literacy: initiatives to improve awareness of how cure research engages with people’s lives

Favorable risk/benefit ratio In many early HIV cure studies, participants will not medically benefit from study interventions • There may be health-related ‘side-benefits’ from being in cure trials • Should side-benefits count in assessment of the risk/benefit relationship? • Are low (or no) benefit, high-risk research studies acceptable? •

Determining risks • Risks of study interventions in each approach • Not all risks known • All risks, not just physical • Identity, Social, Emotional • Determining acceptability of risks • Studies involving serious co-morbidities • Studies with competent adults versus children • Who decides what’s acceptable? • Participants? Ethics committees? Communities?

Risk Determinations • “There may be adverse effects that are presently unknown and unforeseeable… Possible consequences of [intervention] are unknown. It could have no effect or a positive effect… [It] could also possibly cause cancer, or even spread to your reproductive organs and be passed on to any future children you may have. However, to date no such events have been reported… so this risk is still theoretical… a test to monitor this will be run at various times points during the study. ” Language from HIV cure study consent from Henderson GE “The ethics of HIV “cure” research: what can we learn form consent forms? AIDS Research and Human Retroviruses, 2015

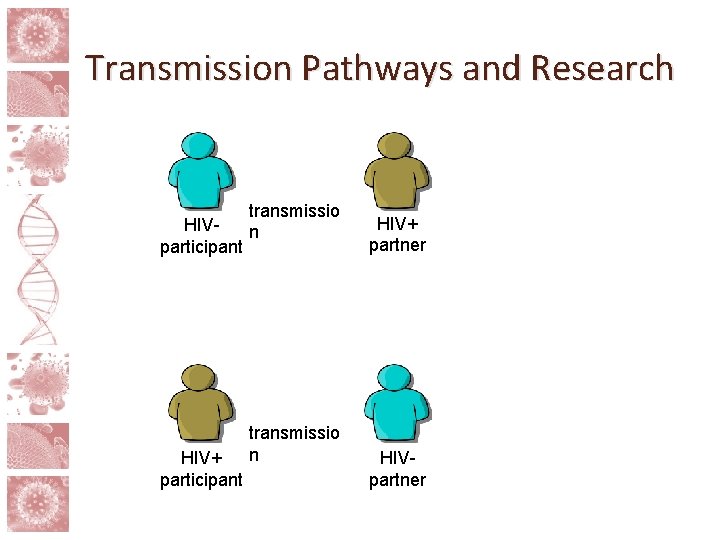
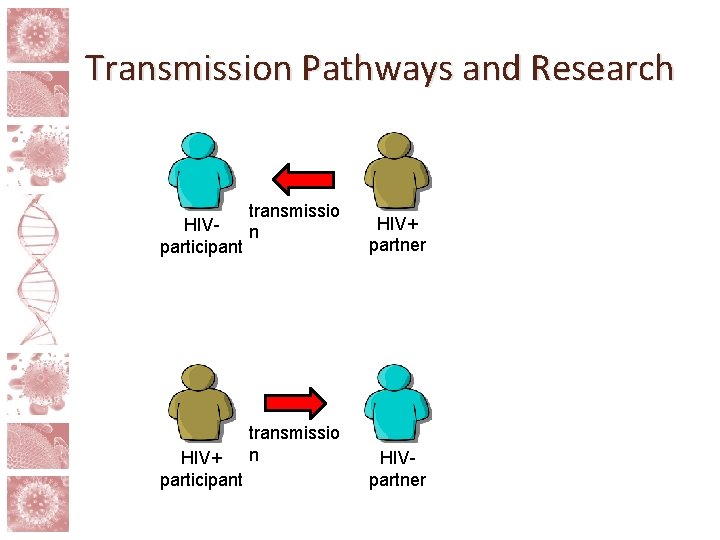
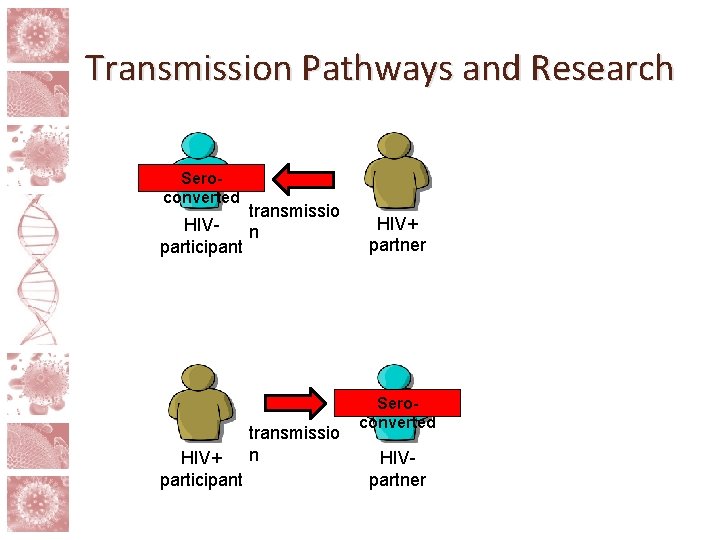
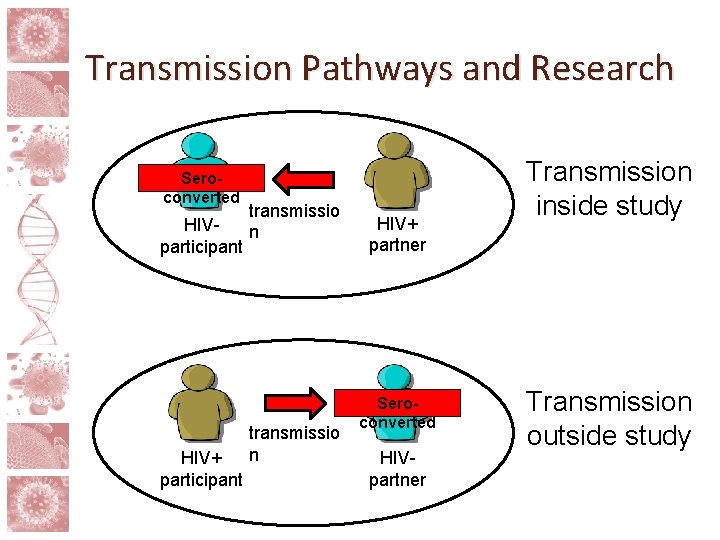
Risk and partner involvement • The risks in HIV cure research go beyond those posed to the participant him/herself • Treatment interruptions and study interventions can raise risk of sexual transmission of HIV • Viral rebound unpredictable, takes time to detect • Possibility of higher risk via behavioral disinhibition • Standard of prevention minus ART

Transmission Pathways and Research transmissio HIVn participant HIV+ participant transmissio n HIV+ partner HIVpartner

Transmission Pathways and Research transmissio HIVn participant HIV+ participant transmissio n HIV+ partner HIVpartner

Transmission Pathways and Research Seroconverted transmissio HIVn participant HIV+ participant transmissio n HIV+ partner Seroconverted HIVpartner

Transmission Pathways and Research Seroconverted transmissio HIVn participant HIV+ participant transmissio n HIV+ partner Seroconverted HIVpartner Transmission inside study Transmission outside study

Ethical highlight: Analytic Treatment Interruption In many studies, HIV+ persons will have to stop ART to test efficacy of curative interventions • Historical resonance: ‘withholding’ standard of care in placebocontrolled HIV prevention studies, i. e. PMTCT • Equipoise: reasonable professional disagreement whether a research intervention is better than current standard of care • Treatment interruption not just different than standard of care, it goes against it • Is there reasonable disagreement whether continued ART or a curative intervention is better for the participant’s health?

Ethics of ATI • Need arguments/safeguards for ATI to be acceptable even if involves potentially harmful departure from standard of care • Selection of participants less likely to be harmed by ATI (i. e. co-morbidities) • Careful monitoring of viral load and follow-up of participants after ATI • Role of altruism in cure research

Current cure research strategies • Latency Reversing Agents • Gene Therapy and Stem Cell Transplantation • Immune Modulators • Early treatment (including pediatric studies)

Latency reversing agents (LRA) • Two step strategies to flush out virus from resting cells and then follow up with an effective killing mechanism HIV DNA

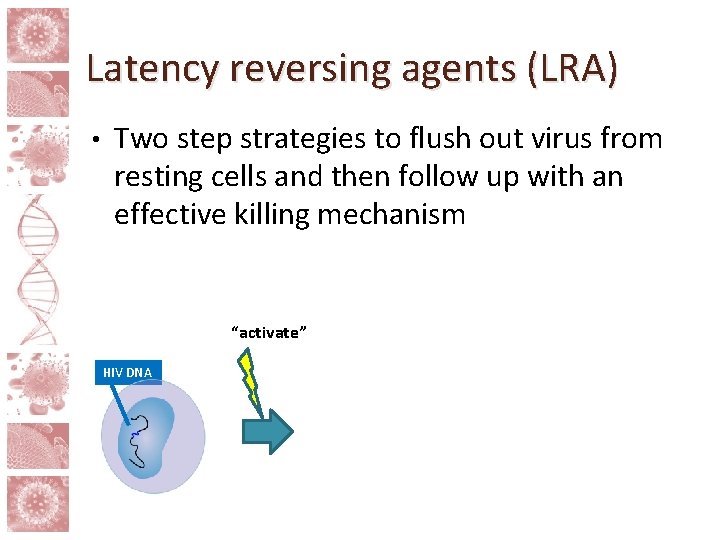
Latency reversing agents (LRA) • Two step strategies to flush out virus from resting cells and then follow up with an effective killing mechanism “activate” HIV DNA

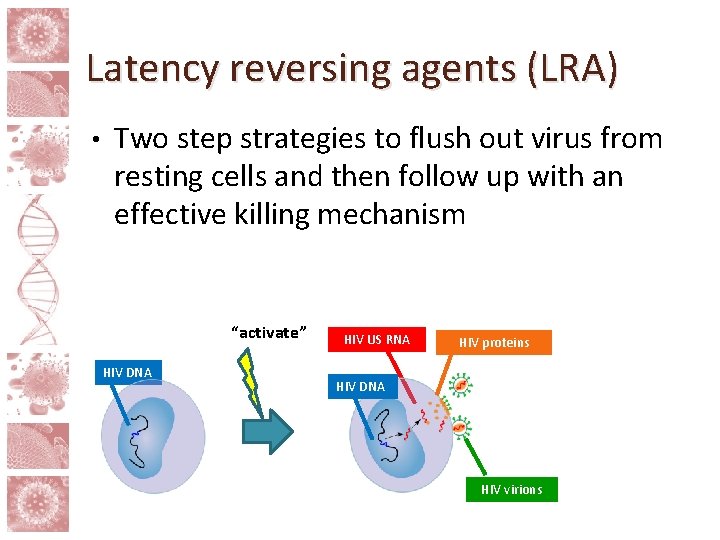
Latency reversing agents (LRA) • Two step strategies to flush out virus from resting cells and then follow up with an effective killing mechanism “activate” HIV DNA HIV US RNA HIV proteins HIV DNA HIV virions

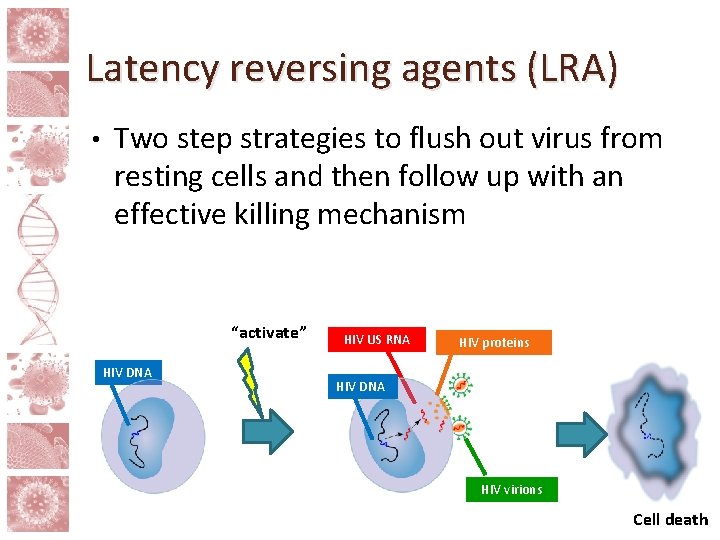
Latency reversing agents (LRA) • Two step strategies to flush out virus from resting cells and then follow up with an effective killing mechanism “activate” HIV DNA HIV US RNA HIV proteins HIV DNA HIV virions Cell death

LRAs- ethical considerations • • • May not require analytical treatment interruption in the short-term But have not been shown to cause a substantial reduction in the size of the replication-competent HIV reservoir to date Possible consequences of reactivating latently infected cells Will not be sufficient alone and will need to be paired with an immune strategy Benefits of research accrue to science and society Ethical considerations of using anti-cancer drugs

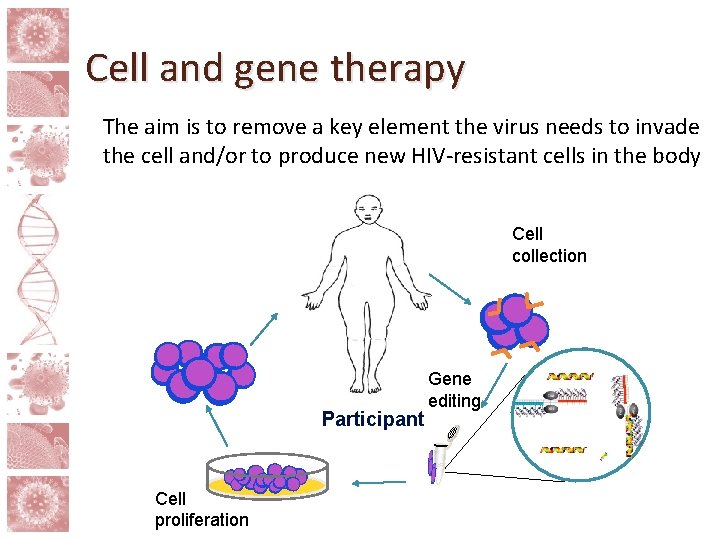
Cell and gene therapy The aim is to remove a key element the virus needs to invade the cell and/or to produce new HIV-resistant cells in the body Cell collection Participant Cell proliferation Gene editing

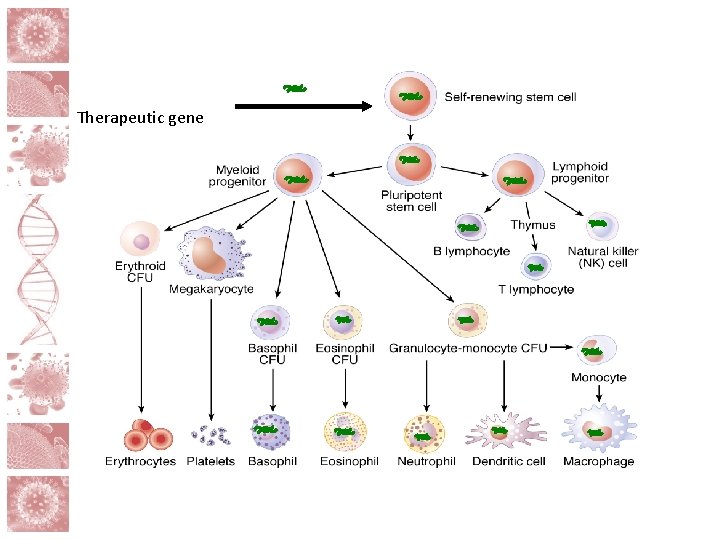
Therapeutic gene

Cell and gene therapy challenges • During cell modification, the percentage of cells modified varies, and a low yield of modified cells is a barrier • Precision is key, a serious concern is “off target” editing

Cell and gene therapy- ethical considerations • Scalability of the approach • Risk to participants who are otherwise healthy • Potential race and clade differences • Likely need to be used in combination

Immune modulators • Class of techniques utilizing the immune system to develop or provide cells with protection to kill HIV • Includes therapeutic vaccines, passive immunization and broadly neutralizing antibodies

Immune modulators – ethical considerations • Development of autoimmunity (risk vs. reward) • Half life • Unlikely to work on their own

Early ART- ethical considerations • Treatment interruption is not medically necessary and potentially harmful • Early treatment is not curative but combination approaches may first be available for those treated early • Treatment fatigue • Consenting issues (pediatric)

Conclusions • Cure research will likely take combination approach like prevention and treatment • HIV cure research is an evolving field and ethical considerations need to be worked through by experts with participant and community collaboration

Module Contributors
 Quasi experimental design ethical issues
Quasi experimental design ethical issues Ethical consideration
Ethical consideration Hiv cure
Hiv cure Writing strategies and ethical considerations
Writing strategies and ethical considerations Qualitative proposal example
Qualitative proposal example Ethical considerations examples
Ethical considerations examples Dissect the broad area into sub-areas
Dissect the broad area into sub-areas Perbedaan ethical dilemma dan ethical lapse
Perbedaan ethical dilemma dan ethical lapse Ethical reasoning model army
Ethical reasoning model army Chapter 4 ethics and social responsibility
Chapter 4 ethics and social responsibility Ethical decision making and ethical leadership
Ethical decision making and ethical leadership What are the general considerations in machine design?
What are the general considerations in machine design? Tax considerations for setting up a new business
Tax considerations for setting up a new business Software architecture of atm machine
Software architecture of atm machine Exchange transaction and relationship in marketing
Exchange transaction and relationship in marketing Mechanical design of transmission line
Mechanical design of transmission line Database design considerations
Database design considerations Contrasting acquisition
Contrasting acquisition Cloud delivery models
Cloud delivery models Chapter 9 pre examination/preanalytical considerations
Chapter 9 pre examination/preanalytical considerations Bioreactor considerations for animal cell culture
Bioreactor considerations for animal cell culture Collaboration design considerations
Collaboration design considerations Mechanical considerations of tooth preparation
Mechanical considerations of tooth preparation Anatomical considerations
Anatomical considerations Biopharmaceutic considerations in drug product design
Biopharmaceutic considerations in drug product design Retromylohyoid space
Retromylohyoid space Videos xxx
Videos xxx Design considerations icon
Design considerations icon Temperature regulation pdhpe
Temperature regulation pdhpe Spurt and shunt muscles
Spurt and shunt muscles Identify the device
Identify the device Design considerations for mobile computing
Design considerations for mobile computing Non rebreather mask definition
Non rebreather mask definition Consideration examples
Consideration examples Web security considerations
Web security considerations Practical considerations for costume design might include
Practical considerations for costume design might include Pricing considerations and approaches
Pricing considerations and approaches Operational considerations definition
Operational considerations definition Azure landing zone considerations
Azure landing zone considerations