REALWORLD CASE STUDY 1 Authoring of CDS Mature

- Slides: 4

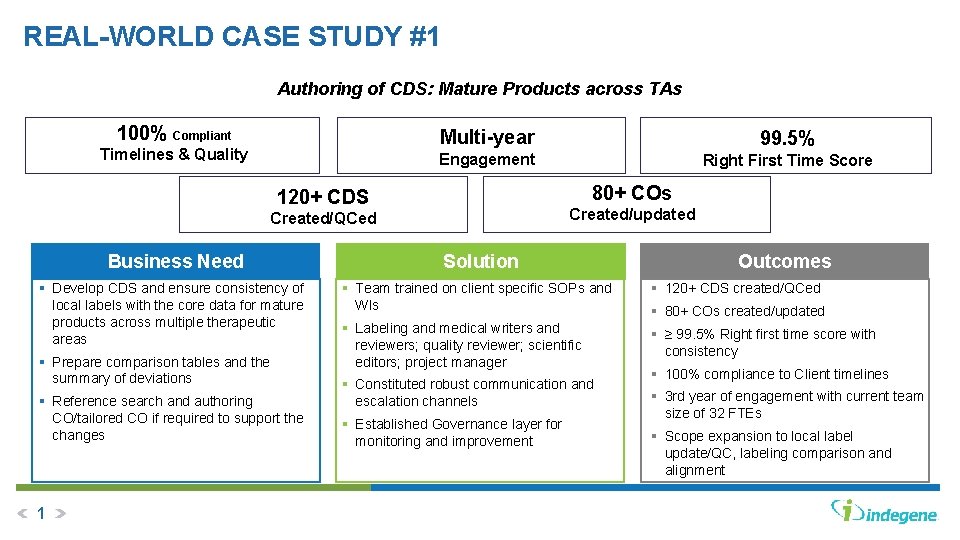
REAL-WORLD CASE STUDY #1 Authoring of CDS: Mature Products across TAs 100% Compliant Timelines & Quality Multi-year 99. 5% Engagement Right First Time Score 80+ COs 120+ CDS Created/updated Created/QCed Business Need § Develop CDS and ensure consistency of local labels with the core data for mature products across multiple therapeutic areas § Prepare comparison tables and the summary of deviations § Reference search and authoring CO/tailored CO if required to support the changes 1 Solution Outcomes § Team trained on client specific SOPs and WIs § 120+ CDS created/QCed § Labeling and medical writers and reviewers; quality reviewer; scientific editors; project manager § ≥ 99. 5% Right first time score with consistency § Constituted robust communication and escalation channels § Established Governance layer for monitoring and improvement § 80+ COs created/updated § 100% compliance to Client timelines § 3 rd year of engagement with current team size of 32 FTEs § Scope expansion to local label update/QC, labeling comparison and alignment

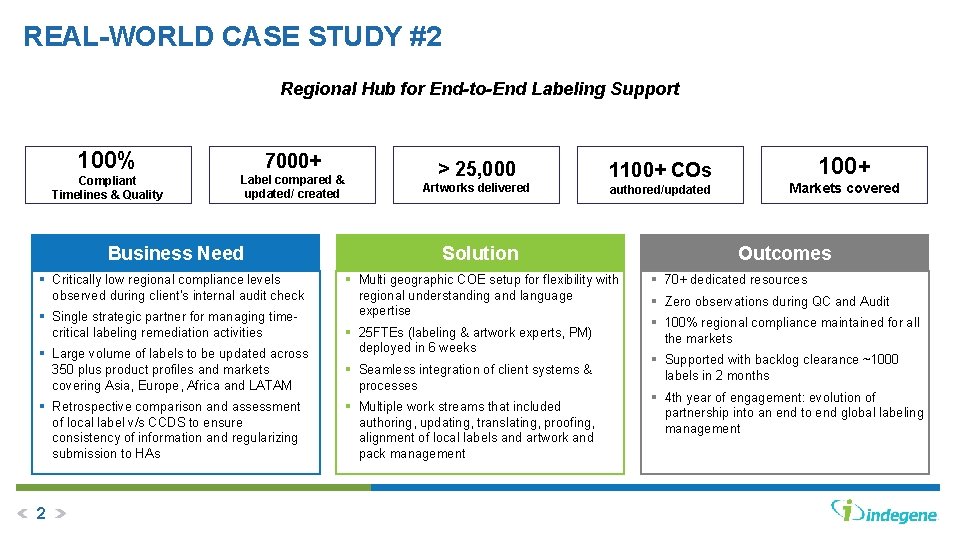
REAL-WORLD CASE STUDY #2 Regional Hub for End-to-End Labeling Support 100% 7000+ Compliant Timelines & Quality Label compared & updated/ created > 25, 000 Artworks delivered 1100+ COs authored/updated Business Need Solution § Critically low regional compliance levels observed during client’s internal audit check § Multi geographic COE setup for flexibility with regional understanding and language expertise § Single strategic partner for managing timecritical labeling remediation activities § Large volume of labels to be updated across 350 plus product profiles and markets covering Asia, Europe, Africa and LATAM § Retrospective comparison and assessment of local label v/s CCDS to ensure consistency of information and regularizing submission to HAs 2 § 25 FTEs (labeling & artwork experts, PM) deployed in 6 weeks § Seamless integration of client systems & processes § Multiple work streams that included authoring, updating, translating, proofing, alignment of local labels and artwork and pack management 100+ Markets covered Outcomes § 70+ dedicated resources § Zero observations during QC and Audit § 100% regional compliance maintained for all the markets § Supported with backlog clearance ~1000 labels in 2 months § 4 th year of engagement: evolution of partnership into an end to end global labeling management

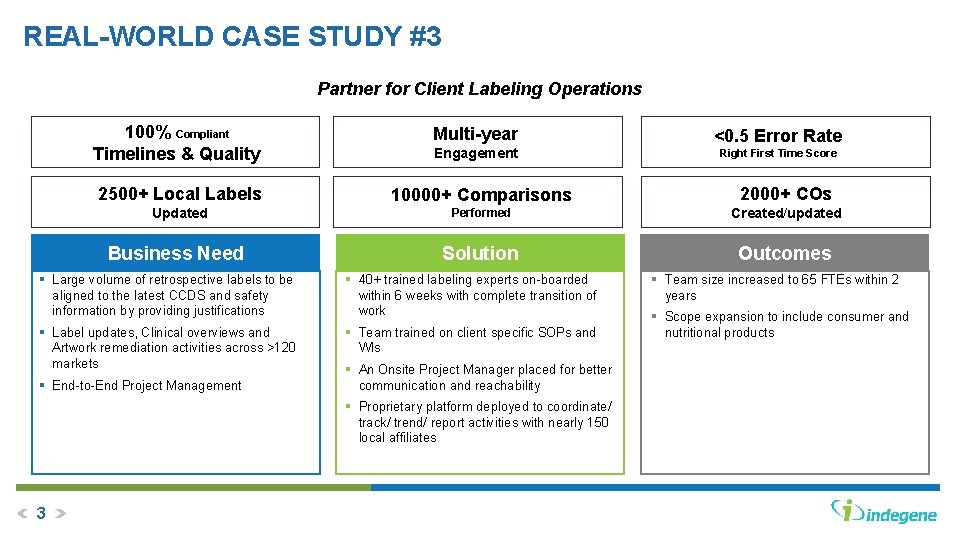
REAL-WORLD CASE STUDY #3 Partner for Client Labeling Operations 100% Compliant Timelines & Quality Multi-year Engagement Right First Time Score 2500+ Local Labels 10000+ Comparisons 2000+ COs Updated Performed Created/updated Solution Outcomes Business Need § Large volume of retrospective labels to be aligned to the latest CCDS and safety information by providing justifications § 40+ trained labeling experts on-boarded within 6 weeks with complete transition of work § Label updates, Clinical overviews and Artwork remediation activities across >120 markets § Team trained on client specific SOPs and WIs § End-to-End Project Management § An Onsite Project Manager placed for better communication and reachability § Proprietary platform deployed to coordinate/ track/ trend/ report activities with nearly 150 local affiliates 3 <0. 5 Error Rate § Team size increased to 65 FTEs within 2 years § Scope expansion to include consumer and nutritional products

REAL-WORLD CASE STUDY #4 Label QC and Proofreading Engagement 100% Regulatory Compliant 2000+ Labels QCed Business Need > 4000 Artworks proofread 1100+ COs authored/updated 99. 9% first time right Solution Outcomes § High-volume local label updates required a QC review to ensure consistency and accuracy § Strategic offshore hubs at Bangalore and Shanghai, leveraging geographical diversity and scale § Workshops with client to stay ahead of global regulatory trends and new labeling technology § Client was looking for cost-effective model to support Quality check, Editorial review and Proof-reading of CDS, Local Product/ Patient Labels and Artwork § Centralized teams of 5 Labeling reviewer and 10 proofreader to manage multiple geographies § Expanded to include multiple other global destinations through a shared-service model § The expected volume to be delivered was >2000 labels and >3000 artworks per year 4 § CDS reviewed for consistent medical information across different sections, consistency in language, style and formatting § 4 th Year of engagement with 3000+ labels proofread per year







