REAL WORLD CASE STUDY RealWorld Case Study 1

- Slides: 7

REAL WORLD CASE STUDY

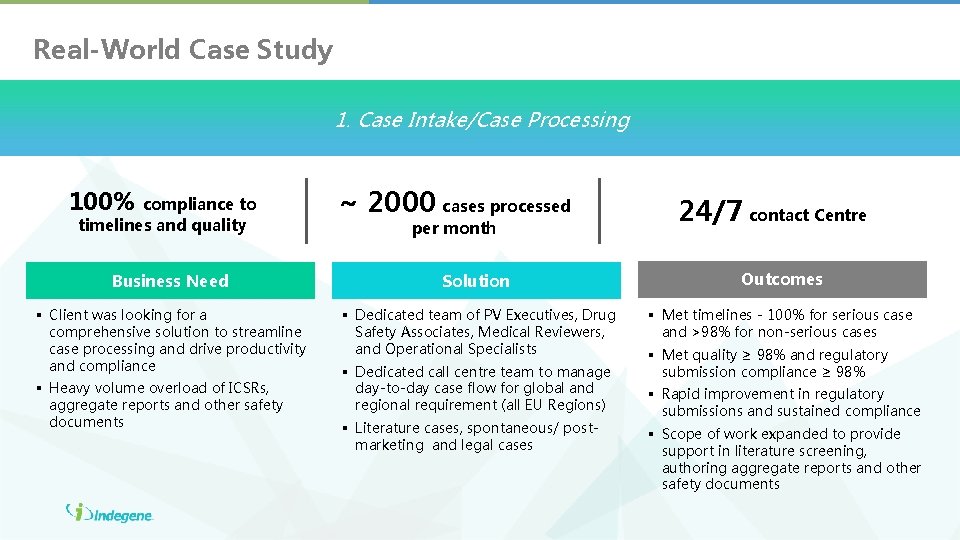
Real-World Case Study 1. Case Intake/Case Processing 100% compliance to timelines and quality ~ 2000 cases processed per month 24/7 contact Centre Business Need Solution Outcomes § Client was looking for a comprehensive solution to streamline case processing and drive productivity and compliance § Dedicated team of PV Executives, Drug Safety Associates, Medical Reviewers, and Operational Specialists § Met timelines - 100% for serious case and >98% for non-serious cases § Heavy volume overload of ICSRs, aggregate reports and other safety documents § Dedicated call centre team to manage day-to-day case flow for global and regional requirement (all EU Regions) § Literature cases, spontaneous/ postmarketing and legal cases § Met quality ≥ 98% and regulatory submission compliance ≥ 98% § Rapid improvement in regulatory submissions and sustained compliance § Scope of work expanded to provide support in literature screening, authoring aggregate reports and other safety documents

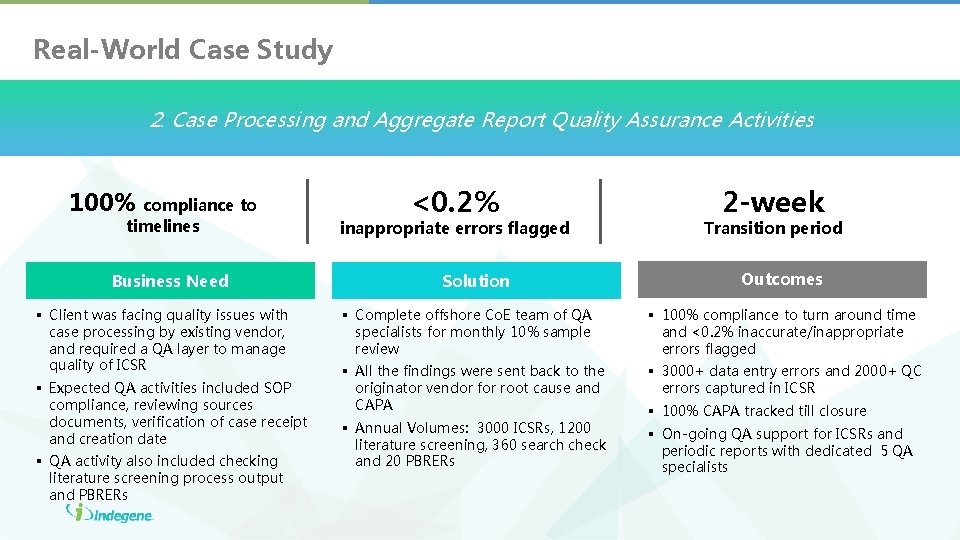
Real-World Case Study 2. Case Processing and Aggregate Report Quality Assurance Activities 100% compliance to timelines Business Need § Client was facing quality issues with case processing by existing vendor, and required a QA layer to manage quality of ICSR § Expected QA activities included SOP compliance, reviewing sources documents, verification of case receipt and creation date § QA activity also included checking literature screening process output and PBRERs <0. 2% inappropriate errors flagged Solution 2 -week Transition period Outcomes § Complete offshore Co. E team of QA specialists for monthly 10% sample review § 100% compliance to turn around time and <0. 2% inaccurate/inappropriate errors flagged § All the findings were sent back to the originator vendor for root cause and CAPA § 3000+ data entry errors and 2000+ QC errors captured in ICSR § Annual Volumes: 3000 ICSRs, 1200 literature screening, 360 search check and 20 PBRERs § On-going QA support for ICSRs and periodic reports with dedicated 5 QA specialists § 100% CAPA tracked till closure

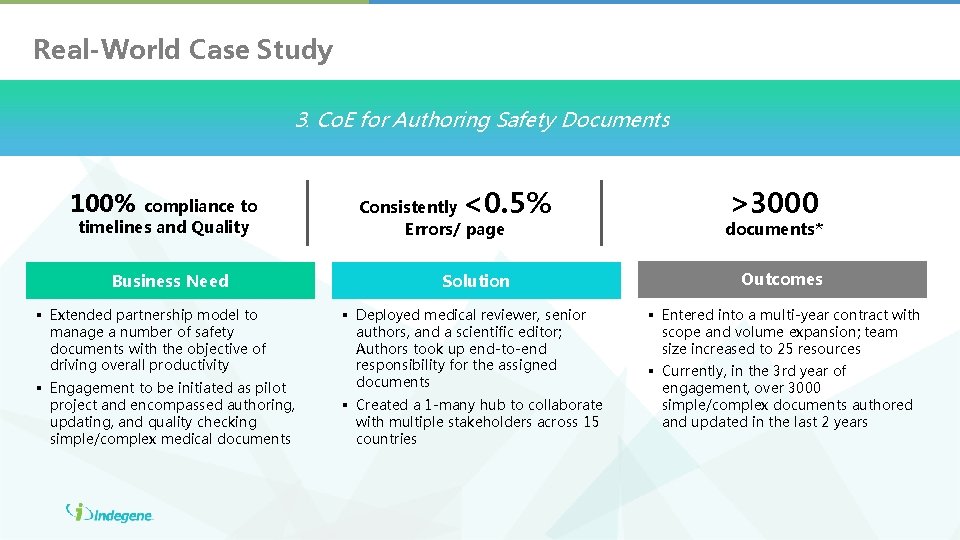
Real-World Case Study 3. Co. E for Authoring Safety Documents 100% compliance to timelines and Quality Business Need § Extended partnership model to manage a number of safety documents with the objective of driving overall productivity § Engagement to be initiated as pilot project and encompassed authoring, updating, and quality checking simple/complex medical documents <0. 5% >3000 Consistently Errors/ page documents* Solution Outcomes § Deployed medical reviewer, senior authors, and a scientific editor; Authors took up end-to-end responsibility for the assigned documents § Created a 1 -many hub to collaborate with multiple stakeholders across 15 countries § Entered into a multi-year contract with scope and volume expansion; team size increased to 25 resources § Currently, in the 3 rd year of engagement, over 3000 simple/complex documents authored and updated in the last 2 years

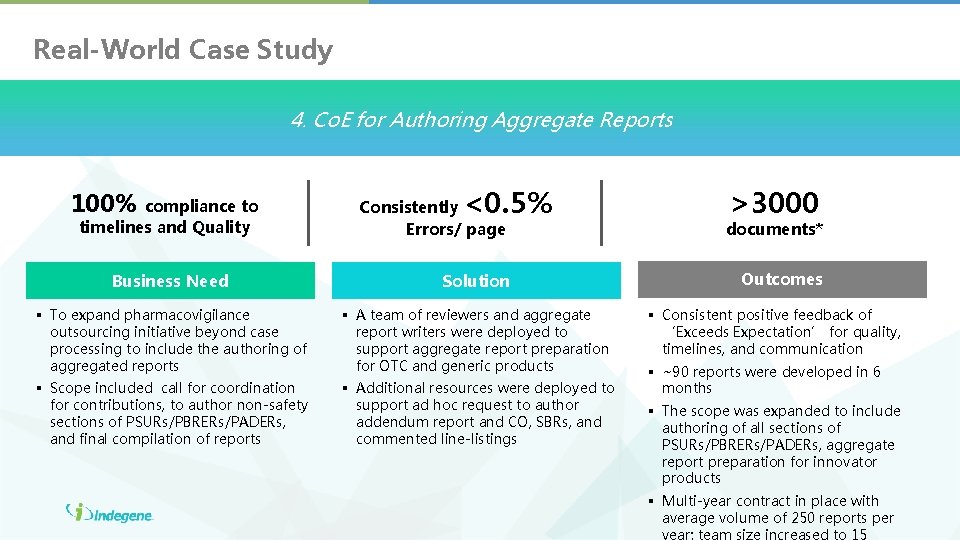
Real-World Case Study 4. Co. E for Authoring Aggregate Reports 100% compliance to timelines and Quality <0. 5% >3000 Consistently Errors/ page documents* Business Need Solution Outcomes § To expand pharmacovigilance outsourcing initiative beyond case processing to include the authoring of aggregated reports § A team of reviewers and aggregate report writers were deployed to support aggregate report preparation for OTC and generic products § Scope included call for coordination for contributions, to author non-safety sections of PSURs/PBRERs/PADERs, and final compilation of reports § Additional resources were deployed to support ad hoc request to author addendum report and CO, SBRs, and commented line-listings § Consistent positive feedback of ‘Exceeds Expectation’ for quality, timelines, and communication § ~90 reports were developed in 6 months § The scope was expanded to include authoring of all sections of PSURs/PBRERs/PADERs, aggregate report preparation for innovator products § Multi-year contract in place with average volume of 250 reports per year; team size increased to 15

Real-World Case Study 5. Risk Management Plan 100% compliance to timelines <0. 5 errors per page ~600 Products managed Solution Outcomes § Develop/ update Risk Management Plans (RMPs) for both innovator and generic products. § Deployed a team of senior authors, safety scientist, medical reviewers and medical literature search associate § Successful collaboration for 3+ years § Preparation of RMP: Aggregate Analysis, Risk-Benefit Assessment, medical analysis and medical writing § Literature screened from multiple external and client databases for risk benefit analysis § Updates to RMPs and REMS Medication guide § Aggregate analysis, risk-benefit assessment and medical analysis was performed by safety scientist and medical reviewer § 75+ HA submission ready RMPs/REMS documents updated/ developed in one year. Business Need § Developed RMP templates and transferred the legacy RMPs with relevant sections updated § Conversion of over 100+ RMPs as per new EU PV legislation in 6 months § Currently, multi-year engagement in place to support authoring and maintenance of RMPs and REMS

Real-World Case Study 6. Safety Data Exchange Agreements Hybrid Delivery model Business Need § HA Inspection identified lack of SDEAs contractual arrangements with business partners § Support required for CAPA measures and roll out ~50 SDEAs in 3 months § Along with CAPA measure, client wanted BAU support in preparation and maintenance of SDEA ~100 SDEAs per year 3 weeks Onsite Client training Solution End-to-end SDEA development work Outcomes § Multi geographic COE setup for flexibility and time zone coverage § Train-the-trainer approach implemented for offshore team. § UK-based Program Director based, with offshore team of Safety Lead and five (5) Safety Associates § Seamless integration with 100% offshore team on-boarded to client system within a week of onsite training § End-to-end SME support to assess master contract and partner PV capabilities, draft SDEAs, negotiate with stakeholders and finalize the SDEA for approval § Also involved in formal announcement of SDEA with effective dates to relevant stakeholders § Communication strategies, project management plan and tracker developed to ensure compliance to timeline § Fortnightly status report and monthly Governance meeting to review the progress