RAM Ready and Motivated Monday 1021 Week 10












- Slides: 15

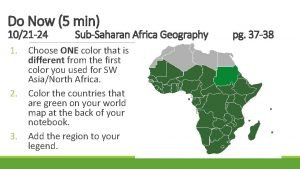
RAM – Ready and Motivated Monday – 10/21 Week 10 Please put this information at the top of your next notebook page Parent-Teacher Conferences Unit 2 – Physical and Chemical Properties of Matter ur o y n i e # t ri age W P 33

RAM – Ready and Motivated Tuesday – 10/22 Week 10 Please put this information at the top of your next notebook page Quiz Substances and Mixtures Unit 2 – Physical and Chemical Properties of Matter Test November 20 ur o y n i e # t ri age W P 33

Standard 7. P. 2: The student will demonstrate an understanding of the structure and properties of matter and that matter is conserved as it undergoes changes. Learning Objective 7. P. 2 B. 1 Analyze and interpret data to describe substances using physical properties (including state, boiling/melting point, density, conductivity, color, hardness, and magnetic properties) and chemical properties (the ability to burn or rust). Activator Tues, 10/22 Page 33 Quiz – Friday Unit 2 Test - Nov. 20

Notes Teacher Directed Instruction Melting Point • The temperature at which a solid can change to a liquid • Unchanging under constant conditions • Example: Ice melts to form liquid water at 0° C (32° F). Boiling Point • The temperature at which a liquid changes from a liquid to a gas. • Boiling begins when bubbles form, grow larger, rise to the surface, and burst. • As long as the substance is boiling the temperature of the liquid remains constant (at the boiling point). • Boiling point is unchanging under constant conditions for a given substance. • Example: The boiling point for pure water at sea level is 100° C (212°F). Tues, 10/22 Page 33 Quiz – Friday Unit 2 Test - Nov. 20

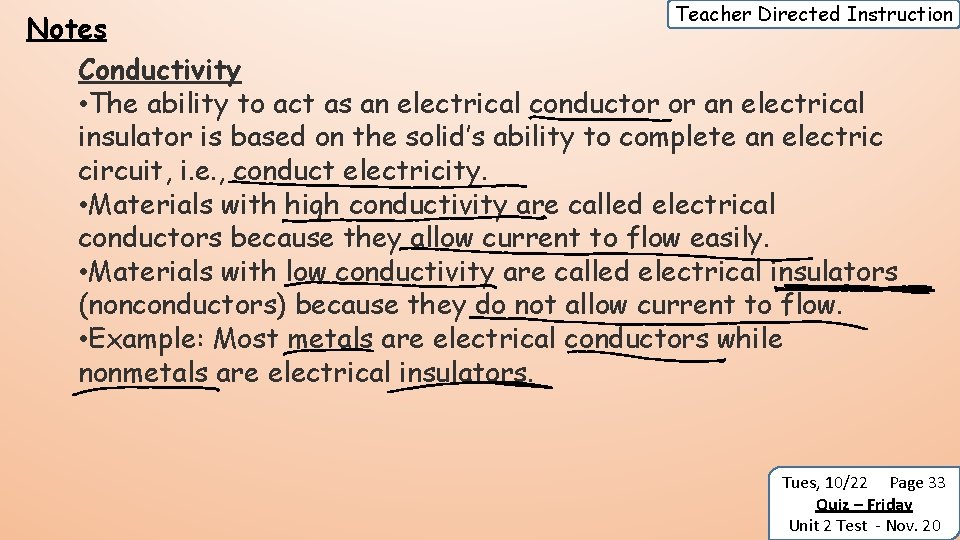
Teacher Directed Instruction Notes Conductivity • The ability to act as an electrical conductor or an electrical insulator is based on the solid’s ability to complete an electric circuit, i. e. , conduct electricity. • Materials with high conductivity are called electrical conductors because they allow current to flow easily. • Materials with low conductivity are called electrical insulators (nonconductors) because they do not allow current to flow. • Example: Most metals are electrical conductors while nonmetals are electrical insulators. Tues, 10/22 Page 33 Quiz – Friday Unit 2 Test - Nov. 20

Notes Teacher Directed Instruction Color • Color can be used to help identify a substance, along with other properties. • By itself, color is not a significant identifier of a substance. • Absence of color is also a physical property. Hardness • The relative resistance of a metal or other material to denting, scratching, or bending. Magnetism • The property of reacting to the forces exerted by magnets • Example: materials that are attracted to magnets are considered to be magnetic. Magnetic materials are mostly metals. Tues, 10/22 Page 33 Quiz – Friday Unit 2 Test - Nov. 20

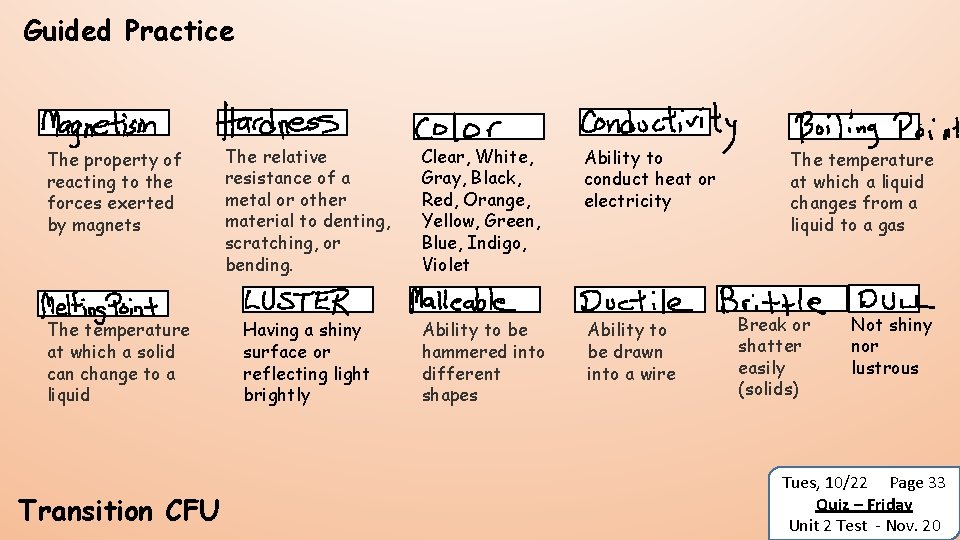
Guided Practice MAGNETISM The property of reacting to the forces exerted by magnets MELTING POINT The temperature at which a solid can change to a liquid Transition CFU HARDNESS The relative resistance of a metal or other material to denting, scratching, or bending. LUSTER Having a shiny surface or reflecting light brightly COLOR Clear, White, Gray, Black, Red, Orange, Yellow, Green, Blue, Indigo, Violet CONDUCTIVITY BOILING POINT Ability to conduct heat or electricity The temperature at which a liquid changes from a liquid to a gas MALLEABLE DUCTILE Ability to be hammered into different shapes Ability to be drawn into a wire BRITTLE Break or shatter easily (solids) DULL Not shiny nor lustrous Tues, 10/22 Page 33 Quiz – Friday Unit 2 Test - Nov. 20

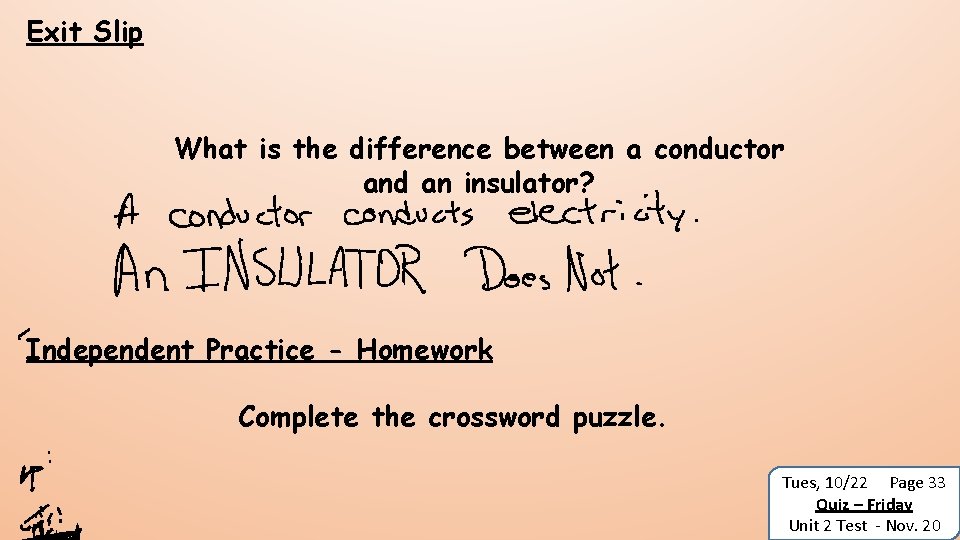
Exit Slip What is the difference between a conductor and an insulator? Independent Practice - Homework Complete the crossword puzzle. Tues, 10/22 Page 33 Quiz – Friday Unit 2 Test - Nov. 20

RAM – Ready and Motivated Wednesday – 10/23 Week 10 Please put this information at the top of your next notebook page Take out your homework and tape it to page 34. Short day for early release and Pep Rally. Unit 2 – Physical and Chemical Properties of Matter ur o y n i e # t ri age W P 34

RAM – Thursday – 10/24 Ready and Motivated Week 10 Please put this information at the top of your next notebook page Mr. Spence out for birth of first grandchild! Complete pages 20 – 25 Turn in for a grade. Unit 2 – Physical and Chemical Properties of Matter ur o y n i e # t ri age W P 35

RAM – Ready and Motivated Friday – 10/25 Week 10 Please put this information at the top of your next notebook page How do you identify a “CHEMICAL” reaction? • • Video Evidence of Chemical Reacions Pearson Chapter 1 Lesson 4 Gas production Color change Change in energy or temperature Formation of a precipitate Unit 2 – Physical and Chemical Properties of Matter ur o y n i e # t ri age W P 34

7. P. 2: The student will demonstrate an understanding of the structure and properties of matter and that matter is conserved as it undergoes changes. Learning Objective 7. P. 2 B. 1 Analyze and interpret data to describe substances using physical properties (including state, boiling/melting point, density, conductivity, color, hardness, and magnetic properties) and chemical properties (the ability to burn or rust). Wed, 10/25 Page 34 Benchmark Wed, 10/30 Unit 2 Test - Nov. 20

Notes Teacher Directed Instruction Density • The relationship between the mass of a material and its volume • Substances that are more dense contain more matter in a given volume. • The density of a substance is unchanging no matter how large or small the sample of the substance. Example: 1. Lead is a very heavy, dense metal. The density of lead is much greater than the density of the very light metal, aluminum. 2. Generally, metals have a heavier density than nonmetals. Fri, 10/25 Page 34 Benchmark Wed 10/30 Unit 2 Test - Nov. 20

Teacher Directed Instruction Notes Chemical properties • can be recognized only when substances react or do not react chemically with one another, that is, when they undergo a change in composition. • cannot be observed without changing the composition of the substance, changing it into a new substance. Fri, 10/23 Page 34 Benchmark Wed 10/30 Unit 2 Test - Nov. 20

Notes Teacher Directed Instruction The following chemical properties can be used to help identify a substance: • Ability to burn: The ability to burn involves a substance reacting quickly with oxygen to produce light and heat. This process is called burning • Ability to rust: The ability of a substance to rust is a chemical property that involves a substance reacting slowly with oxygen. The process is called rusting. Fri 10/25 Page 34 Benchmark Wed 10/30 Unit 2 Test - Nov. 20
 1021/24
1021/24 Ram nam me lin hai dekhat sabme ram
Ram nam me lin hai dekhat sabme ram Stay ready so you don't have to get ready
Stay ready so you don't have to get ready Monday my favorite day
Monday my favorite day Moday tuesday
Moday tuesday Week by week plans for documenting children's development
Week by week plans for documenting children's development Motivated energized and capable faculty
Motivated energized and capable faculty Motivated system 2 reasoning
Motivated system 2 reasoning Hawthorne effect in business
Hawthorne effect in business Knowdell card sorts
Knowdell card sorts Motivated reasoning
Motivated reasoning The 3 gs of exploration
The 3 gs of exploration Are the sought-after results of motivated behavior
Are the sought-after results of motivated behavior Define motivated forgetting
Define motivated forgetting Monroe's motivated sequence example
Monroe's motivated sequence example When are capabilities-motivated acquisitions essential?
When are capabilities-motivated acquisitions essential?