PROSPERO International prospective register of systematic reviews An









- Slides: 15

PROSPERO International prospective register of systematic reviews An overview of development and progress May 2013 Centre for Reviews and Dissemination

Background: the need for registration • Systematic reviews usually provide the evidence base upon which health and social care decisions are made so they should be robust and free from bias • There is concern about publication and selective outcome reporting biases associated with systematic reviews • Unplanned duplication of reviews is a waste of resource – No open register for review protocols (Cochrane and Campbell Collaboration protocol registration limited to their own organisations) • PRISMA 2009 Checklist: asks for protocol and registration details Centre for Reviews and Dissemination

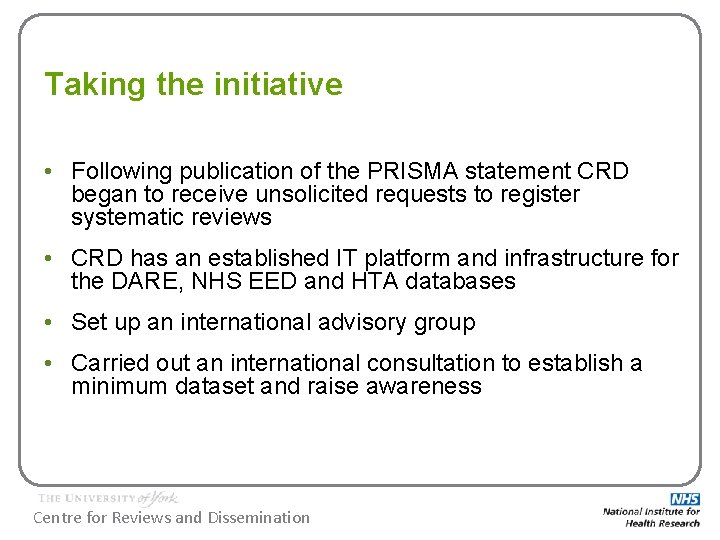
Taking the initiative • Following publication of the PRISMA statement CRD began to receive unsolicited requests to register systematic reviews • CRD has an established IT platform and infrastructure for the DARE, NHS EED and HTA databases • Set up an international advisory group • Carried out an international consultation to establish a minimum dataset and raise awareness Centre for Reviews and Dissemination

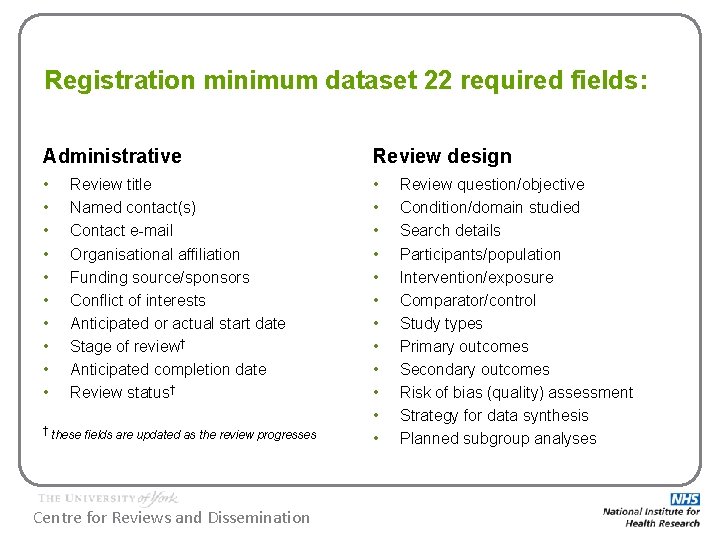
Registration minimum dataset 22 required fields: Administrative Review design • • • • • • Review title Named contact(s) Contact e-mail Organisational affiliation Funding source/sponsors Conflict of interests Anticipated or actual start date Stage of review† Anticipated completion date Review status† † these fields are updated as the review progresses Centre for Reviews and Dissemination Review question/objective Condition/domain studied Search details Participants/population Intervention/exposure Comparator/control Study types Primary outcomes Secondary outcomes Risk of bias (quality) assessment Strategy for data synthesis Planned subgroup analyses

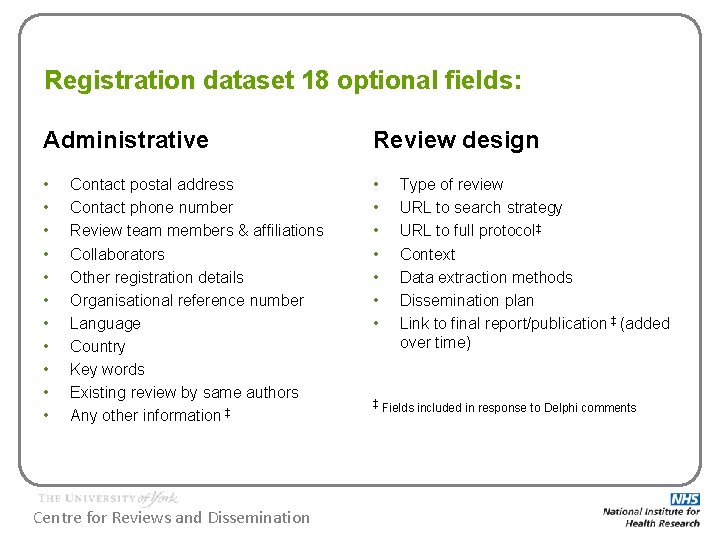
Registration dataset 18 optional fields: Administrative Review design • • • • • Contact postal address Contact phone number Review team members & affiliations Collaborators Other registration details Organisational reference number Language Country Key words Existing review by same authors Any other information ‡ Centre for Reviews and Dissemination Type of review URL to search strategy URL to full protocol‡ Context Data extraction methods Dissemination plan Link to final report/publication ‡ (added over time) ‡ Fields included in response to Delphi comments

PROSPERO: International prospective register of systematic reviews • • Web based Free to register Free to search Users create and update their own records Minimum data set required Record content is the responsibility of review lead Administrators check for “sense” not peer review A public audit trail of amendments is maintained Centre for Reviews and Dissemination

Eligibility for inclusion in PROSPERO • Systematic reviews of the effects of interventions and strategies to prevent, diagnose, treat, and monitor health conditions, for which there is a health related outcome • Reviews of methodological issues need to contain at least one outcome of direct patient or clinical relevance in order to be included in PROSPERO (included from November 2012) • Systematic reviews of reviews with a health related outcome (included from November 2012) • New Cochrane review protocols uploaded automatically (should not be registered individually) (included from Jan 2013) • Registration should take place once the systematic review protocol is finalised, but ideally before screening studies for inclusion begins Centre for Reviews and Dissemination

Eligibility for inclusion in PROSPERO • Exclusions: – Scoping reviews – reviews of animal studies – reviews of methods for which there is no direct health related outcome • Reviews that have progressed beyond the completion of data extraction are not accepted • Completed reviews are not accepted Centre for Reviews and Dissemination

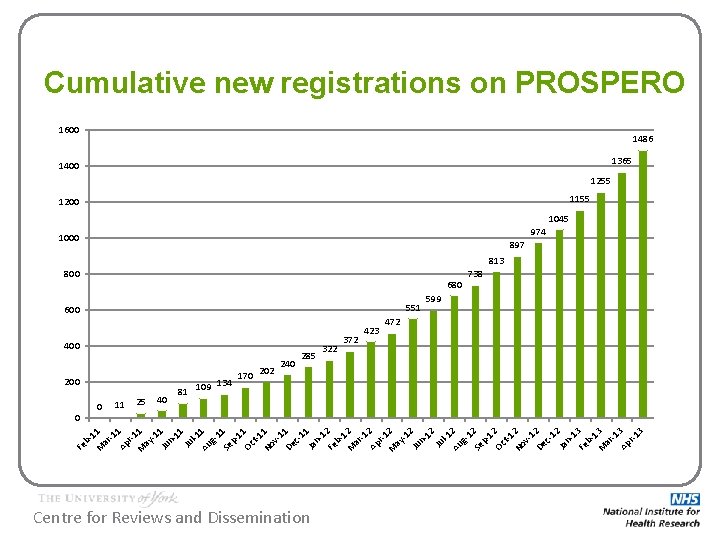
The story so far… PROSPERO opened for registration 22 February 2011 In April 2013: • Registered reviews being undertaken in 57 different countries • 1467 registrations published: – – 1245 are ongoing reviews 167 are completed but not yet published 62 are completed and published 6 have been abandoned Centre for Reviews and Dissemination

Cumulative new registrations on PROSPERO 1600 1486 1365 1400 1255 1155 1200 1045 974 1000 897 813 800 680 551 600 400 200 11 ar 11 81 202 M b. Fe 40 170 285 372 599 472 -1 Ap 1 r-1 M 1 ay -1 1 Ju n 11 Ju l-1 Au 1 g 1 Se 1 p 11 Oc t-1 No 1 v 1 De 1 c 11 Ja n 1 Fe 2 b 1 M 2 ar -1 Ap 2 r-1 M 2 ay -1 2 Ju n 12 Ju l-1 Au 2 g 1 Se 2 p 12 Oc t-1 No 2 v 1 De 2 c 12 Ja n 1 Fe 3 b 1 M 3 ar -1 Ap 3 r-1 3 0 0 25 109 134 240 322 423 738 Centre for Reviews and Dissemination

Supporters of the principle of registration and PROSPERO • Organisations and networks – NIHR, Cochrane and Campbell Collaborations, Joanna Briggs Institute, INAHTA, Guidelines International Network, James Lind Alliance, ANZCTR, WHO Alliance for Health Policy and Systems Research, AZQ, Public Health Wales, HNODS • Publishers – PLo. S journals, BMJ Open, Bio. Med Central, BJOG: recommend registration in their instructions to authors • Funders – The UK NIHR have introduced mandatory registration for all the eligible systematic reviews they fund Centre for Reviews and Dissemination

Achieving aims • Reduce unplanned duplication of reviews: – the NIHR HTA Programme decided not to commission research on restricted elimination diet in Attention Deficit. Hyperactivity Disorder after finding on PROSPERO an overlapping research project already in progress • Allow comparison of plans with final report: – PROSPERO registration number now appearing in final reports in peer reviewed journals – Peer reviewers are contacting CRD with queries, in particular about stage of review at time of acceptance Centre for Reviews and Dissemination

Evaluation of utility at one year Administrative data and web statistics were collated an online survey of users’ experiences undertaken: • Registration is feasible and not overly burdensome • On the whole, survey respondents are satisfied the system allows registration in a straightforward and acceptable way • Some changes introduced to improve user experience e. g. save draft as editable document Centre for Reviews and Dissemination

Future developments • Encourage more supporters to help raise awareness of the value of prospective registration of systematic reviews • Work with commissioning and funding organisations to help facilitate grant holder uptake and compliance • Continue to broaden the inclusion criteria • Promote the wide use of both the registration process and the search facility • Conduct methodological research Centre for Reviews and Dissemination

The development and ongoing management of PROSPERO is supported by CRD’s core work programme which is funded by the National Institute for Health Research, England; the Department of Health, Public Health Agency, Northern Ireland the National Institute for Social Care and Health Research, Welsh Government The views expressed are those of the author(s) and not necessarily those of the NHS, NIHR, Department of Health, or any of CRD’s funders www. crd. york. ac. uk/PROSPERO Centre for Reviews and Dissemination
 Prospero registration
Prospero registration Prospero systematic review
Prospero systematic review Prospero register
Prospero register Riverside permit portal
Riverside permit portal Why do prince prospero and his followers retreat
Why do prince prospero and his followers retreat Prospero
Prospero What task does prospero give ferdinand
What task does prospero give ferdinand Prospero and caliban relationship
Prospero and caliban relationship The masque of the red death setting
The masque of the red death setting What does prince prospero symbolize
What does prince prospero symbolize Sciglass
Sciglass Retrospective causal-comparative research
Retrospective causal-comparative research The persistence of learning over time
The persistence of learning over time Prospective memory psychology definition
Prospective memory psychology definition Longitudinal prospective study
Longitudinal prospective study Prospective analysis financial statements
Prospective analysis financial statements