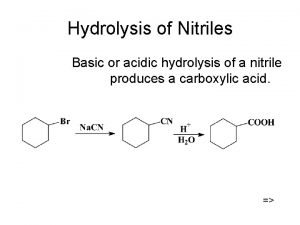
Predicting the p H of salt solutions Hydrolysis





- Slides: 9

Predicting the p. H of salt solutions

Hydrolysis of ions Hydrolysis refers to a reaction with water (e. g. splitting water into H+ and OH–) When salts are added to water, p. H can change E. g. when Na 3 PO 4 is added to water, ions form Na 3 PO 4(aq) 3 Na+(aq) + PO 43–(aq) These ions may react with H 2 O, affecting the p. H PO 43–(aq) + H+(aq) HPO 42–(aq) Na+(aq) + OH–(aq) Na. OH (aq) If the anion (-ve) reacts to remove lots of H+ but the cation (+ve) removes very little OH–, then H+ will decrease and the solution will be basic.

The degree of hydrolysis • • • PO 43–(aq) + H+(aq) HPO 42–(aq) Na+ + OH– Na. OH The problem with writing equilibria this way is we do not know the strength of the reactions However, if we reverse the reaction we can look up Ka and Kb values (pg. 608, 615): – 13 2– 3– + Ka= 4. 5 x 10 HPO 4 + H Kb= 55 Na. OH Na+ + OH– Small Ka: few products; adding PO 43– = shift left Large Kb: mostly products; Na+ has little affect Thus, adding Na 3 PO 4 will cause more H+ to be removed, resulting in a basic solution

Accuracy of predictions Theoretically, using Ka and Kb values you could predict the exact p. H resulting from a certain salt being added to distilled water. However, you only need to be able to predict if a solution will be acidic, basic, or neutral. Note: you can’t judge the p. H change solely on the difference between Ka and Kb. Other factors are involved (e. g. the formula of the compound and its molar mass both affect [ ]) Note: hydrolysis refers to reactions with water. Several variations for writing equilibriums exist. However, focusing on how the H+/OH– balance of water is affected is easiest.

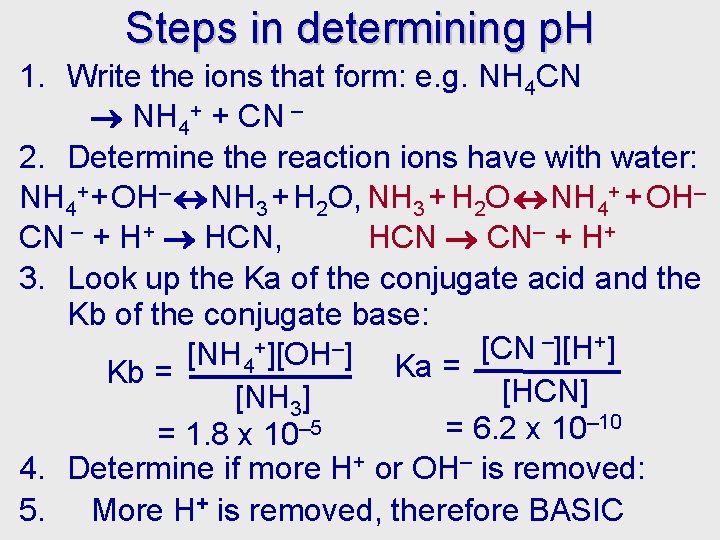
Steps in determining p. H 1. Write the ions that form: e. g. NH 4 CN NH 4+ + CN – 2. Determine the reaction ions have with water: NH 4++ OH– NH 3 + H 2 O, NH 3 + H 2 O NH 4+ + OH– CN – + H+ HCN, HCN CN– + H+ 3. Look up the Ka of the conjugate acid and the Kb of the conjugate base: –][H+] + – [CN [NH 4 ][OH ] Ka = Kb = [HCN] [NH 3] – 10 – 5 = 6. 2 x 10 = 1. 8 x 10 4. Determine if more H+ or OH– is removed: 5. More H+ is removed, therefore BASIC

Buffers - lab Read 15. 6 (621 -623) up to and including special topic 15. 2 (carbonate buffer) Calibrate p. H meter, get a plastic bottle with distilled H 2 O to rinse your p. H meter btw tests You will use 4 solutions ( 20 m. L of each): distilled water, water + Na. C 2 H 3 O 2 (5 scoops), 0. 2 M HC 2 H 3 O 2 + Na. C 2 H 3 O 2 For each, record the initial p. H and the p. H upon addition of 5, 10, and 15 drops of 1 M HCl Remake the 4 solutions For each, record the initial p. H and the p. H upon addition of 5, 10, and 15 drops of 1 M Na. OH

HCl 0 5 10 15 Na OH 0 5 10 15 H 2 O Na. C 2 H 3 O 2 HC 2 H 3 O 2 Na. C 2 H 3 O 2 + HC 2 H 3 O 2

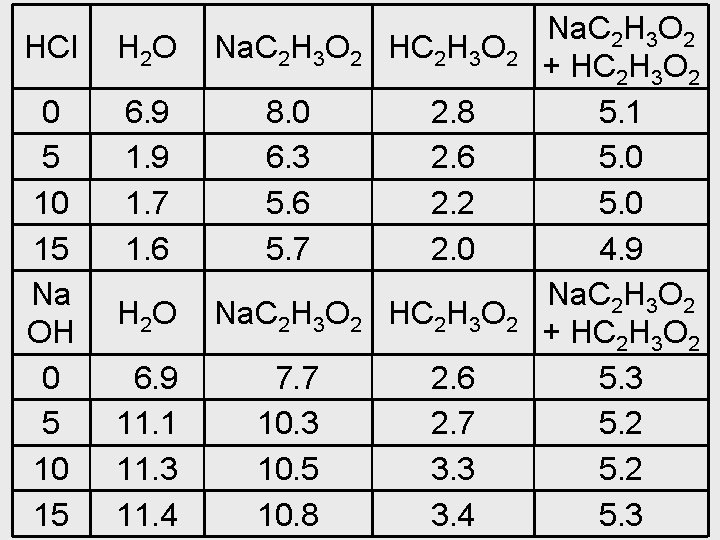
HCl H 2 O 0 5 10 15 Na OH 0 5 10 15 6. 9 1. 7 1. 6 H 2 O 6. 9 11. 1 11. 3 11. 4 Na. C 2 H 3 O 2 HC 2 H 3 O 2 8. 0 6. 3 5. 6 5. 7 2. 8 2. 6 2. 2 2. 0 Na. C 2 H 3 O 2 HC 2 H 3 O 2 7. 7 10. 3 10. 5 10. 8 2. 6 2. 7 3. 3 3. 4 Na. C 2 H 3 O 2 + HC 2 H 3 O 2 5. 1 5. 0 4. 9 Na. C 2 H 3 O 2 + HC 2 H 3 O 2 5. 3 5. 2 5. 3

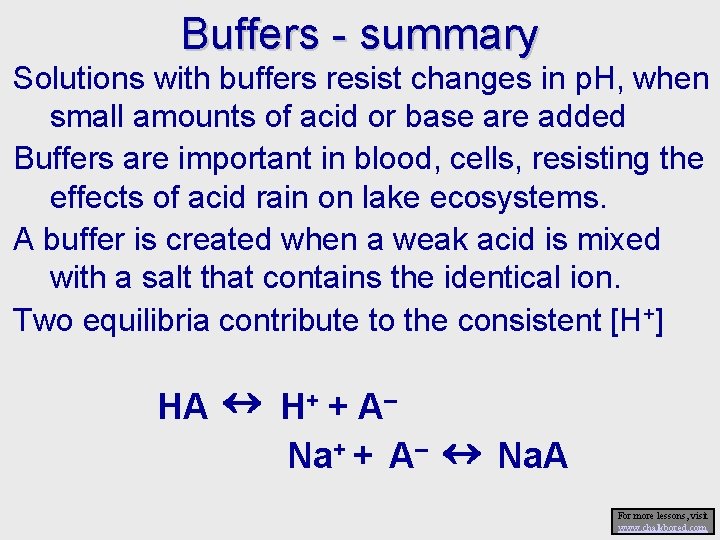
Buffers - summary Solutions with buffers resist changes in p. H, when small amounts of acid or base are added Buffers are important in blood, cells, resisting the effects of acid rain on lake ecosystems. A buffer is created when a weak acid is mixed with a salt that contains the identical ion. Two equilibria contribute to the consistent [H+] HA H+ + A– Na+ + A– Na. A For more lessons, visit www. chalkbored. com