Practicality and Misapplications of Composite Endpoints William S



![SYNTAX one-year Results [NEJM 5 Mar 2009] N MACCE (primary) Death Stroke MI Death/MI/Stroke SYNTAX one-year Results [NEJM 5 Mar 2009] N MACCE (primary) Death Stroke MI Death/MI/Stroke](https://slidetodoc.com/presentation_image_h2/b0cd0e35dda18943b95afdb87bc178ab/image-6.jpg)
![SYNTAX trial 5 -year Outcomes [Lancet 2013; 381 p 629 -] CABG v PCI SYNTAX trial 5 -year Outcomes [Lancet 2013; 381 p 629 -] CABG v PCI](https://slidetodoc.com/presentation_image_h2/b0cd0e35dda18943b95afdb87bc178ab/image-7.jpg)

![ACUITY trial in ACS 30 day outcomes [NEJM 2006; 355 p 2203 -] Heparin ACUITY trial in ACS 30 day outcomes [NEJM 2006; 355 p 2203 -] Heparin](https://slidetodoc.com/presentation_image_h2/b0cd0e35dda18943b95afdb87bc178ab/image-12.jpg)

![HORIZONS-AMI trial [NEJM 7 May 2009] Primary efficacy: target lesion revascularization at 1 year HORIZONS-AMI trial [NEJM 7 May 2009] Primary efficacy: target lesion revascularization at 1 year](https://slidetodoc.com/presentation_image_h2/b0cd0e35dda18943b95afdb87bc178ab/image-14.jpg)
![OPTIMIZE trial [JAMA online 31 Oct 2013] 3 vs 12 months dual antiplatelet therapy OPTIMIZE trial [JAMA online 31 Oct 2013] 3 vs 12 months dual antiplatelet therapy](https://slidetodoc.com/presentation_image_h2/b0cd0e35dda18943b95afdb87bc178ab/image-15.jpg)




- Slides: 19

Practicality and Misapplications of Composite Endpoints William S. Weintraub, MD, MACC, FAHA, FESC Med. Star Washington Hospital Center Washington, DC USA

Practicality and Misapplications of Composite Endpoints William S. Weintraub, MD, MACC, FAHA, FESC Med. Star Washington Hospital Center Washington, DC USA Disclosures: None

Composite Endpoints We need them individual outcomes lack statistical power We hate them components vary in their clinical importance treatment effect varies across components We abuse them they oversimplify a more complex pattern Always explore the details need results for each component 3

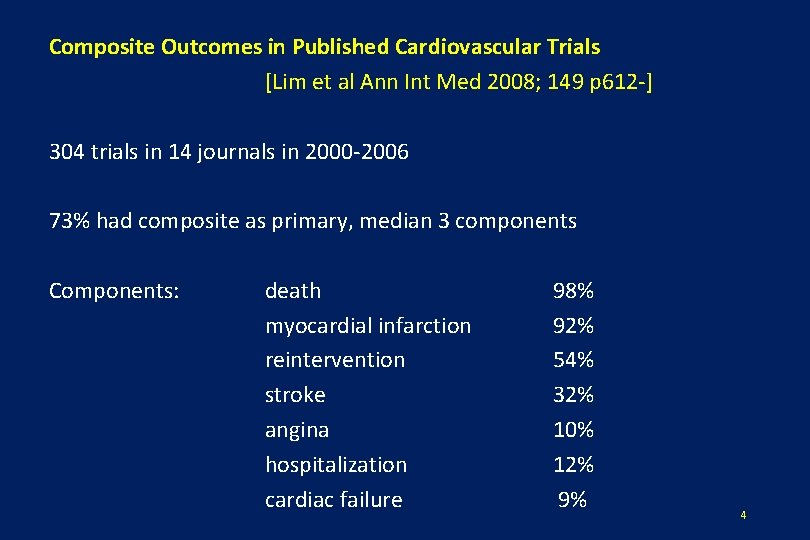
Composite Outcomes in Published Cardiovascular Trials [Lim et al Ann Int Med 2008; 149 p 612 -] 304 trials in 14 journals in 2000 -2006 73% had composite as primary, median 3 components Components: death myocardial infarction reintervention stroke angina hospitalization cardiac failure 98% 92% 54% 32% 10% 12% 9% 4

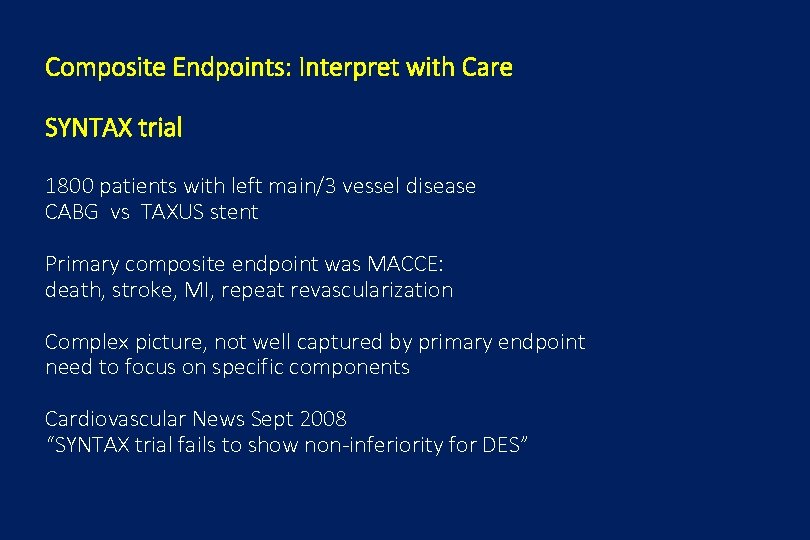
Composite Endpoints: Interpret with Care SYNTAX trial 1800 patients with left main/3 vessel disease CABG vs TAXUS stent Primary composite endpoint was MACCE: death, stroke, MI, repeat revascularization Complex picture, not well captured by primary endpoint need to focus on specific components Cardiovascular News Sept 2008 “SYNTAX trial fails to show non-inferiority for DES”
![SYNTAX oneyear Results NEJM 5 Mar 2009 N MACCE primary Death Stroke MI DeathMIStroke SYNTAX one-year Results [NEJM 5 Mar 2009] N MACCE (primary) Death Stroke MI Death/MI/Stroke](https://slidetodoc.com/presentation_image_h2/b0cd0e35dda18943b95afdb87bc178ab/image-6.jpg)
SYNTAX one-year Results [NEJM 5 Mar 2009] N MACCE (primary) Death Stroke MI Death/MI/Stroke Revascularization PCI CABG 897 105 30 19 28 65 50 40 11 TAXUS 903 159 39 5 43 68 120 102 25 A complex picture, not well captured by primary endpoint P=. 0015 P=. 003 P=. 98 P<. 0001
![SYNTAX trial 5 year Outcomes Lancet 2013 381 p 629 CABG v PCI SYNTAX trial 5 -year Outcomes [Lancet 2013; 381 p 629 -] CABG v PCI](https://slidetodoc.com/presentation_image_h2/b0cd0e35dda18943b95afdb87bc178ab/image-7.jpg)
SYNTAX trial 5 -year Outcomes [Lancet 2013; 381 p 629 -] CABG v PCI in 1800 patients with 3 vessel or left main Death Myocardial infarction Stroke Composite PCI (N=903 13. 9% 9. 7% 3. 7% 20. 8% CABG (N=897) 11. 4% 3. 8% 2. 4% 16. 7% P=. 10 P<. 0001 P=. 09 P=. 03 7

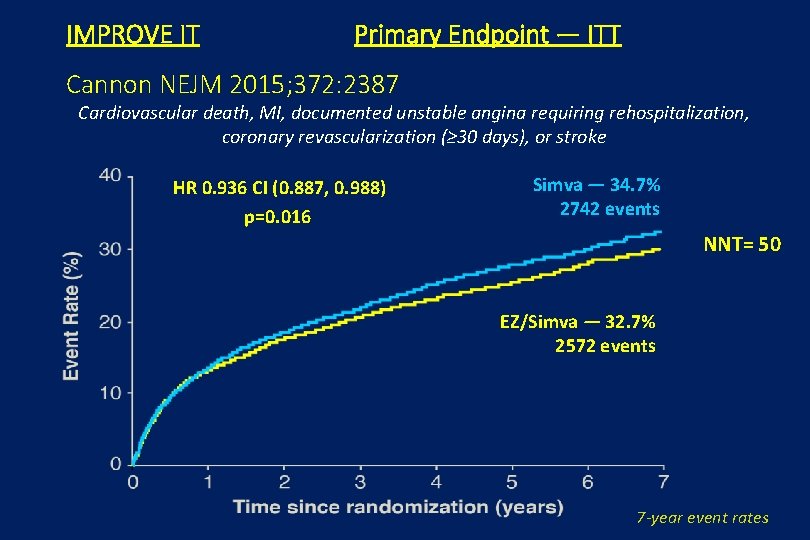
IMPROVE IT Primary Endpoint — ITT Cannon NEJM 2015; 372: 2387 Cardiovascular death, MI, documented unstable angina requiring rehospitalization, coronary revascularization (≥ 30 days), or stroke HR 0. 936 CI (0. 887, 0. 988) p=0. 016 Simva — 34. 7% 2742 events NNT= 50 EZ/Simva — 32. 7% 2572 events 7 -year event rates

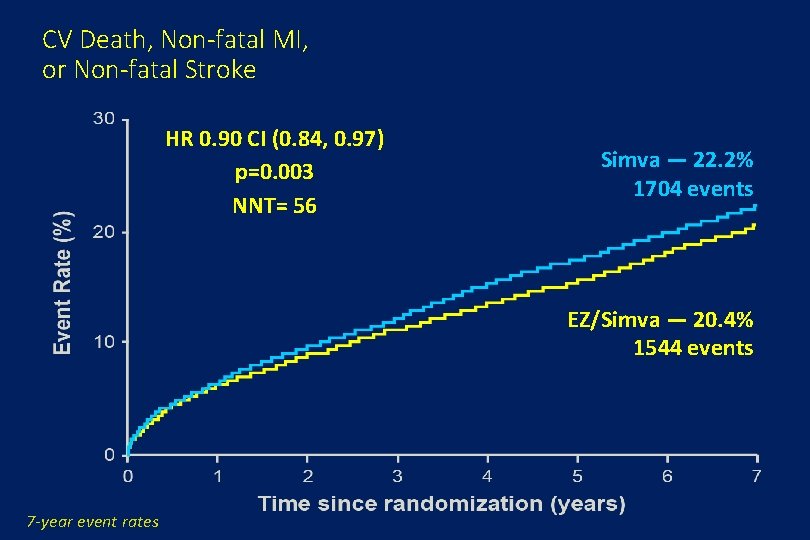
CV Death, Non-fatal MI, or Non-fatal Stroke HR 0. 90 CI (0. 84, 0. 97) p=0. 003 NNT= 56 Simva — 22. 2% 1704 events EZ/Simva — 20. 4% 1544 events 7 -year event rates

Adding “softer” events to the composite: • Makes it harder to interpret • Less clinically meaningful • May or may not enhance statistical power

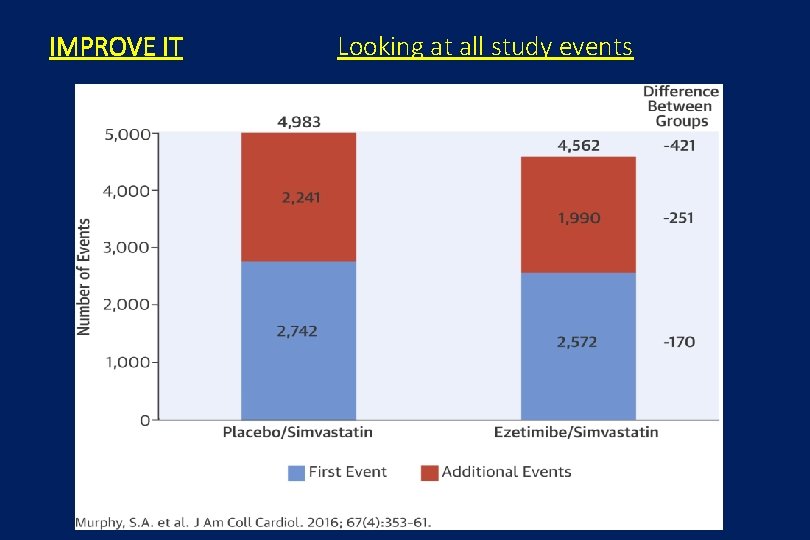
IMPROVE IT Looking at all study events
![ACUITY trial in ACS 30 day outcomes NEJM 2006 355 p 2203 Heparin ACUITY trial in ACS 30 day outcomes [NEJM 2006; 355 p 2203 -] Heparin](https://slidetodoc.com/presentation_image_h2/b0cd0e35dda18943b95afdb87bc178ab/image-12.jpg)
ACUITY trial in ACS 30 day outcomes [NEJM 2006; 355 p 2203 -] Heparin vs + IIb/IIIa inhibitor Bivalirudin alone Composite ischemia (death, MI, revasc. ) 7. 3% 7. 8% RR 1. 08 95% CI 0. 93 to 1. 24 Major bleed 5. 7% 3. 0% P<0. 001 RR 0. 53 95% CI 0. 43 to 0. 65 Net clinical outcome 11. 7% 10. 1% RR 0. 86 95% CI 0. 77 to 0. 97 P=. 015

Net adverse clinical events: Pre-defined composite of efficacy and safety outcomes An objective summary, but: Do components differ in their importance? Primary endpoint or a secondary descriptor?
![HORIZONSAMI trial NEJM 7 May 2009 Primary efficacy target lesion revascularization at 1 year HORIZONS-AMI trial [NEJM 7 May 2009] Primary efficacy: target lesion revascularization at 1 year](https://slidetodoc.com/presentation_image_h2/b0cd0e35dda18943b95afdb87bc178ab/image-14.jpg)
HORIZONS-AMI trial [NEJM 7 May 2009] Primary efficacy: target lesion revascularization at 1 year Composite safety: death, reinfarction, stroke, stent thrombosis TLR Composite safety TAXUS bare-metal stent (N=2257) (N=749) 4. 5% 7. 5% 8. 1% 8. 0% Separates re-intervention from major clinical concerns Non-inferior for safety, components “equally flat” P=. 002 P=. 92
![OPTIMIZE trial JAMA online 31 Oct 2013 3 vs 12 months dual antiplatelet therapy OPTIMIZE trial [JAMA online 31 Oct 2013] 3 vs 12 months dual antiplatelet therapy](https://slidetodoc.com/presentation_image_h2/b0cd0e35dda18943b95afdb87bc178ab/image-15.jpg)
OPTIMIZE trial [JAMA online 31 Oct 2013] 3 vs 12 months dual antiplatelet therapy after drug-eluting stent Primary endpoint: net adverse clinical and cerebral events ie NACCE=death, MI, stroke or major bleed at 1 year expected 9%, non-inferiority margin 2. 7% treatment duration 3 months [N=1563] NACCE: at 1 year : 3 m to 1 yr stent thrombosis major bleeding 93 54 4 3 12 months risk difference (95% CI) [N=1556] 90 52 1 6 Trade-off between small risk of stent thrombosis and small benefit of less bleeding Trial too small to answer that question 0. 2%(-1. 5% to 1. 9%) 0. 1%(-1. 2% to 1. 4%)

Methods for composite endpoints, which give priority to the more serious component e. g. CV death has priority over HF hospitalisation Buyse “Generalised pairwise comparisons of prioritised outcomes in the two-sample problem” [Stats in Med 2010; 30 p 3245 -] Pocock et al “The win ratio: a new approach to the analysis of composite endpoints in clinical trials based on clinical priorities” [Eur Heart J 2012; 33 p 176 -] matched pairs and unmatched approach

The Win Ratio: unmatched approach applied to EMPHASIS trial of eplerenone primary endpoint: CV death or HF hosp compare every eplerenone patient with every placebo patient 1364 x 1371 = 1, 872, 722 unmatched pairs who had CV death first , if not known who had HF hosp first CV death on eplerenone first 124825 HF hosp on eplerenone first 86127 tied 1323085 HF hosp on placebo first 175606 CV death on placebo first 163129 Win ratio = 175606 + 163129 124825 + 86127 eplerenone “losers” eplerenone “winners” = 1. 61 (95% CI 1. 37, 1. 89)

Conclusions: • Composite endpoints can lead to erroneous conclusions • Studies should be designed with the dangers well understood • Similarly, reading of the literature needs to be careful and well informed

Future Directions for Composite Endpoints: • Wiser choice of appropriate composites • Consider the components carefully • Conventional methods (i. e. time to first event) will continue to dominate • Repeat event methods important in chronic disease • Innovation methods based on clinical priorities may prove useful