Pooled 3 year SYMPLICITY HTN1 And SYMPLICITY HTN2


- Slides: 11

Pooled 3 -year SYMPLICITY HTN-1 And SYMPLICITY HTN-2 Results And Diabetes Subgroup Analysis Richard Katholi, M. D Prairie Cardiovascular, Springfield, IL US On behalf of Murray D. Esler, Henry Krum, Krishna Rocha-Singh, Markus P. Schlaich, Michael Böhm, Felix Mahfoud, and the SYMPLICITY HTN Investigators

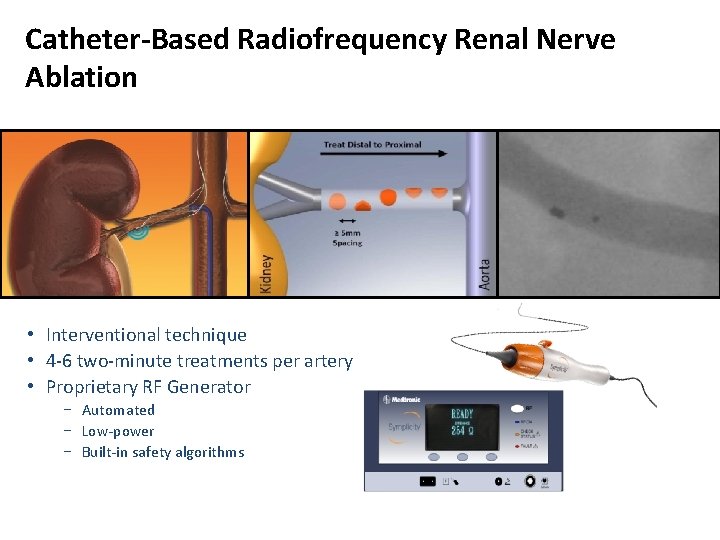
Catheter-Based Radiofrequency Renal Nerve Ablation • Interventional technique • 4 -6 two-minute treatments per artery • Proprietary RF Generator − Automated − Low-power − Built-in safety algorithms

SYMPLICITY HTN-1 and SYMPLICITY HTN-2: Study Designs SYMPLICITY HTN-1: 1 Prospective, International, Multicenter, Nonrandomized, Open-Label Study Patients with Resistant Hypertension § § § Renal denervation with standard of care treatment* (N=153) § Mean age: 57 years Mean baseline BP: 176/98 mm Hg Mean no. antihypertensive medications: 5. 1 § Primary efficacy measure: Change in office BP Primary safety assessments: Physical examination, blood chemistries; anatomic assessment of renal vasculature SYMPLICITY HTN-2: 2 Prospective, International, Multicenter, Randomized Controlled Trial § § § Mean age: 58 years Mean baseline BP: 178/97 mm Hg Mean no. antihypertensive medications: 5. 2 Renal denervation with standard of care treatment* (n=52) Randomization Patients with Resistant Hypertension Primary efficacy measure: Change in office SBP at 6 months† Follow up to 24 months (RDN and Crossover control)3 Maintain previous treatment alone (n=54) *Standard of care treatment consisted of continuation of previous antihypertensive medications †Control patients offered RDN following 6 month primary endpoint BP=blood pressure; SBP=systolic blood pressure. 1. Symplicity HTN-1 Investigators. Hypertension. 2011; 57: 911 -917; 2. Symplicity HTN-2 Investigators. Lancet. 2010; 376: 1903 -1909. 3. Krum H ACC 2013. Caution: The Symplicity™ Renal Denervation System is an Investigational Device. Limited by U. S. law to investigational use. Trademarks may be registered and are the property of their respective owners. For OMA distribution only. © 2014 Medtronic, Inc. All rights reserved. 10132474 DOC 02/2014

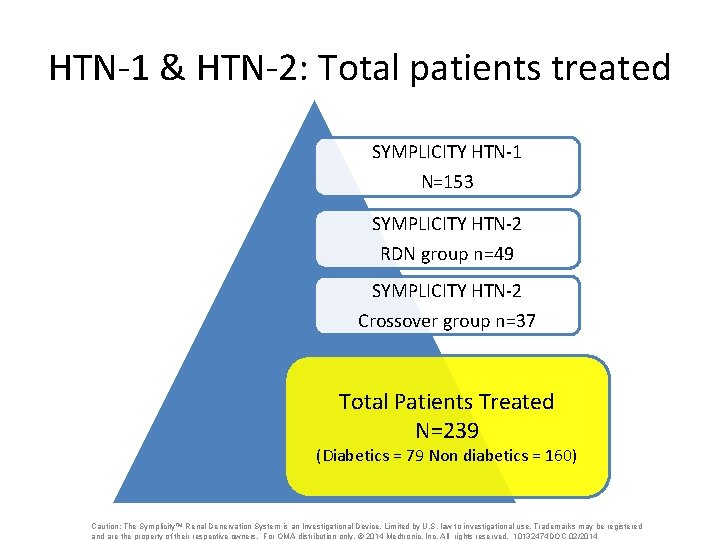
HTN-1 & HTN-2: Total patients treated SYMPLICITY HTN-1 N=153 SYMPLICITY HTN-2 RDN group n=49 SYMPLICITY HTN-2 Crossover group n=37 Total Patients Treated N=239 (Diabetics = 79 Non diabetics = 160) Caution: The Symplicity™ Renal Denervation System is an Investigational Device. Limited by U. S. law to investigational use. Trademarks may be registered and are the property of their respective owners. For OMA distribution only. © 2014 Medtronic, Inc. All rights reserved. 10132474 DOC 02/2014

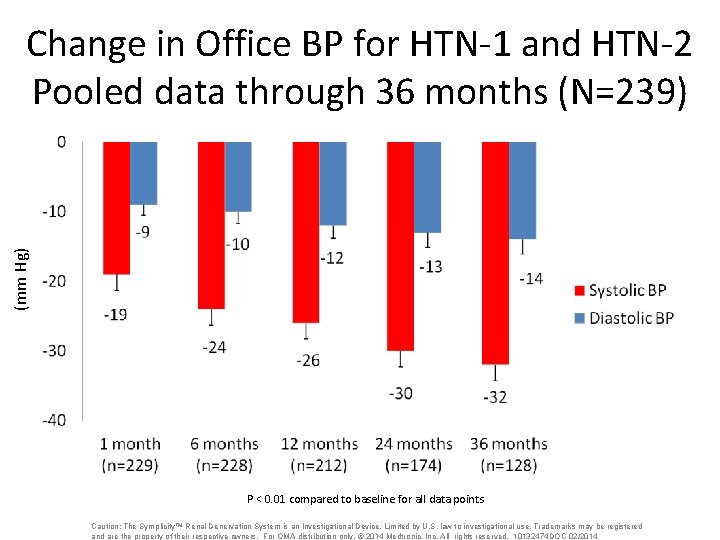
(mm Hg) Change in Office BP for HTN-1 and HTN-2 Pooled data through 36 months (N=239) P < 0. 01 compared to baseline for all data points Caution: The Symplicity™ Renal Denervation System is an Investigational Device. Limited by U. S. law to investigational use. Trademarks may be registered and are the property of their respective owners. For OMA distribution only. © 2014 Medtronic, Inc. All rights reserved. 10132474 DOC 02/2014

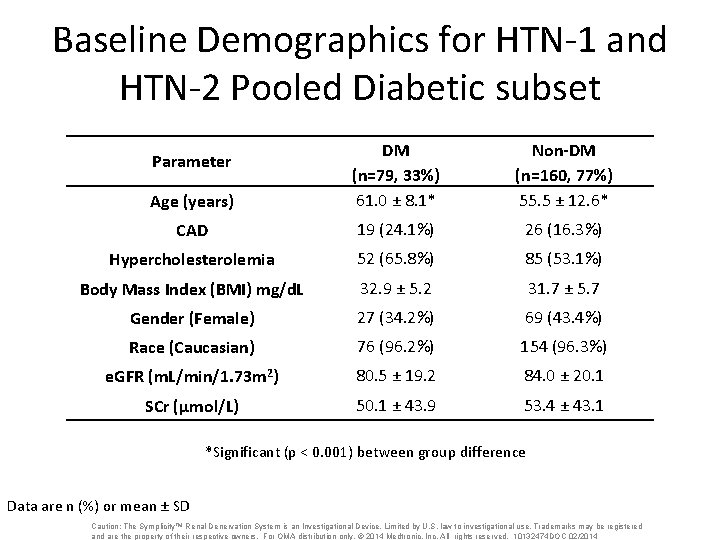
Baseline Demographics for HTN-1 and HTN-2 Pooled Diabetic subset Age (years) DM (n=79, 33%) 61. 0 ± 8. 1* Non-DM (n=160, 77%) 55. 5 ± 12. 6* CAD 19 (24. 1%) 26 (16. 3%) Hypercholesterolemia 52 (65. 8%) 85 (53. 1%) Body Mass Index (BMI) mg/d. L 32. 9 ± 5. 2 31. 7 ± 5. 7 Gender (Female) 27 (34. 2%) 69 (43. 4%) Race (Caucasian) 76 (96. 2%) 154 (96. 3%) e. GFR (m. L/min/1. 73 m 2) 80. 5 ± 19. 2 84. 0 ± 20. 1 SCr (μmol/L) 50. 1 ± 43. 9 53. 4 ± 43. 1 Parameter *Significant (p < 0. 001) between group difference Data are n (%) or mean ± SD Caution: The Symplicity™ Renal Denervation System is an Investigational Device. Limited by U. S. law to investigational use. Trademarks may be registered and are the property of their respective owners. For OMA distribution only. © 2014 Medtronic, Inc. All rights reserved. 10132474 DOC 02/2014

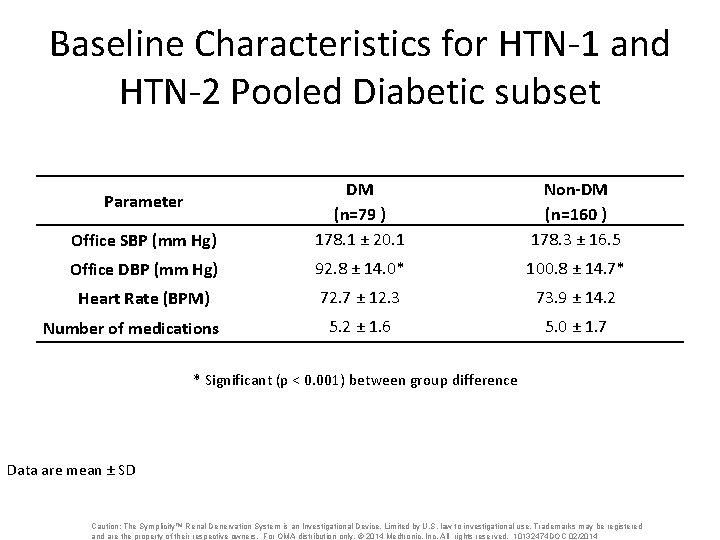
Baseline Characteristics for HTN-1 and HTN-2 Pooled Diabetic subset Office SBP (mm Hg) DM (n=79 ) 178. 1 ± 20. 1 Non-DM (n=160 ) 178. 3 ± 16. 5 Office DBP (mm Hg) 92. 8 ± 14. 0* 100. 8 ± 14. 7* Heart Rate (BPM) 72. 7 ± 12. 3 73. 9 ± 14. 2 5. 2 ± 1. 6 5. 0 ± 1. 7 Parameter Number of medications * Significant (p < 0. 001) between group difference Data are mean ± SD Caution: The Symplicity™ Renal Denervation System is an Investigational Device. Limited by U. S. law to investigational use. Trademarks may be registered and are the property of their respective owners. For OMA distribution only. © 2014 Medtronic, Inc. All rights reserved. 10132474 DOC 02/2014

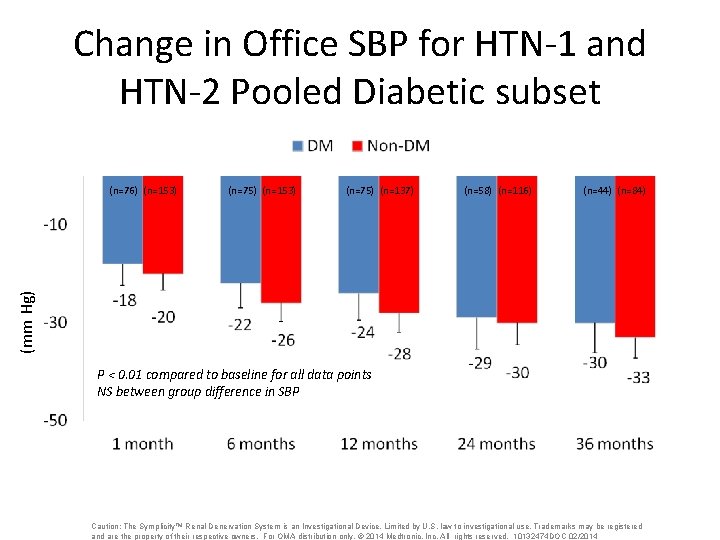
Change in Office SBP for HTN-1 and HTN-2 Pooled Diabetic subset (n=75) (n=153) (n=75) (n=137) (n=58) (n=116) (n=44) (n=84) (mm Hg) (n=76) (n=153) P < 0. 01 compared to baseline for all data points NS between group difference in SBP Caution: The Symplicity™ Renal Denervation System is an Investigational Device. Limited by U. S. law to investigational use. Trademarks may be registered and are the property of their respective owners. For OMA distribution only. © 2014 Medtronic, Inc. All rights reserved. 10132474 DOC 02/2014

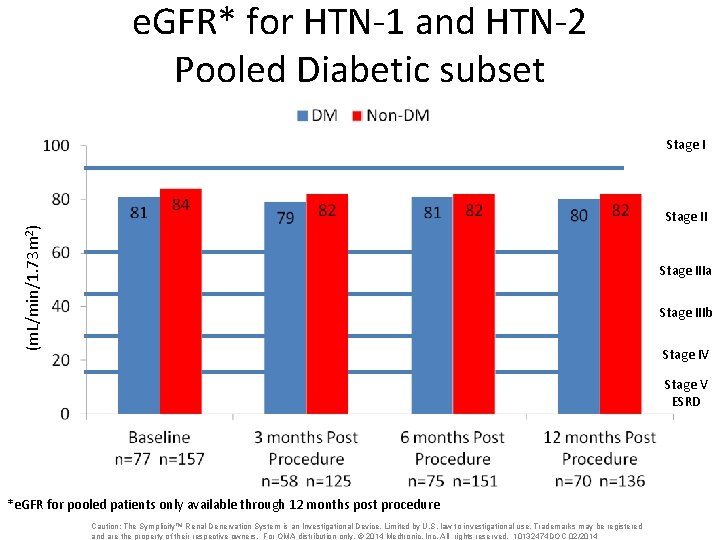
e. GFR* for HTN-1 and HTN-2 Pooled Diabetic subset Stage I (m. L/min/1. 73 m 2) Stage IIIa Stage IIIb Stage IV Stage V ESRD *e. GFR for pooled patients only available through 12 months post procedure Caution: The Symplicity™ Renal Denervation System is an Investigational Device. Limited by U. S. law to investigational use. Trademarks may be registered and are the property of their respective owners. For OMA distribution only. © 2014 Medtronic, Inc. All rights reserved. 10132474 DOC 02/2014

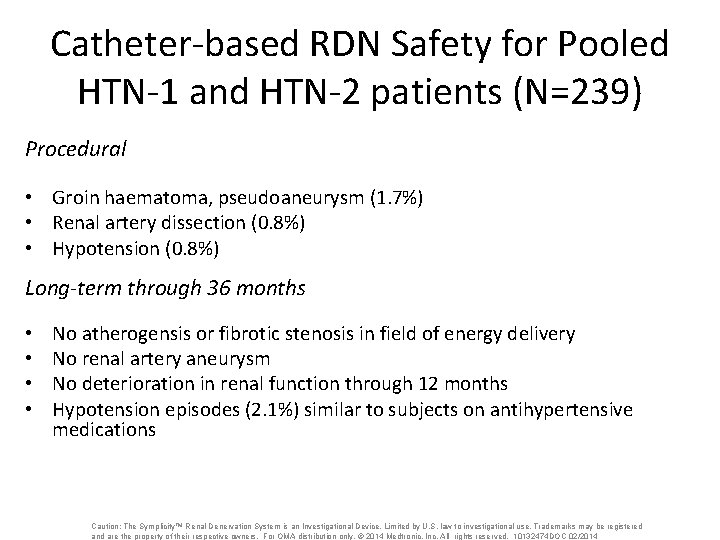
Catheter-based RDN Safety for Pooled HTN-1 and HTN-2 patients (N=239) Procedural • Groin haematoma, pseudoaneurysm (1. 7%) • Renal artery dissection (0. 8%) • Hypotension (0. 8%) Long-term through 36 months • • No atherogensis or fibrotic stenosis in field of energy delivery No renal artery aneurysm No deterioration in renal function through 12 months Hypotension episodes (2. 1%) similar to subjects on antihypertensive medications Caution: The Symplicity™ Renal Denervation System is an Investigational Device. Limited by U. S. law to investigational use. Trademarks may be registered and are the property of their respective owners. For OMA distribution only. © 2014 Medtronic, Inc. All rights reserved. 10132474 DOC 02/2014

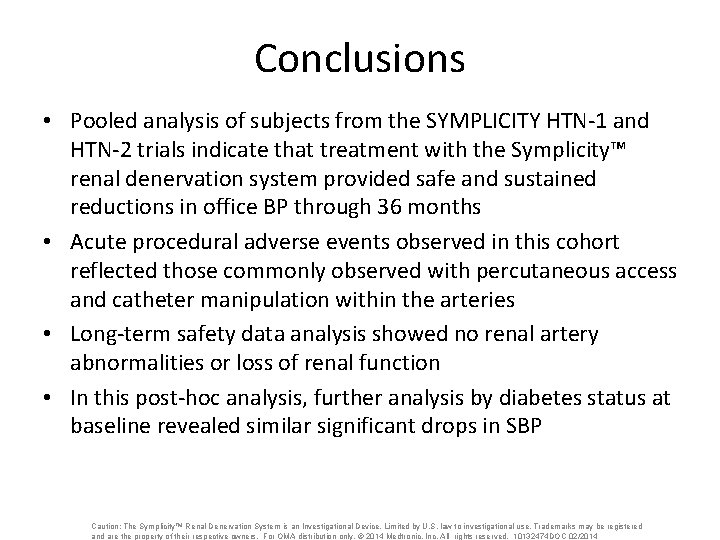
Conclusions • Pooled analysis of subjects from the SYMPLICITY HTN-1 and HTN-2 trials indicate that treatment with the Symplicity™ renal denervation system provided safe and sustained reductions in office BP through 36 months • Acute procedural adverse events observed in this cohort reflected those commonly observed with percutaneous access and catheter manipulation within the arteries • Long-term safety data analysis showed no renal artery abnormalities or loss of renal function • In this post-hoc analysis, further analysis by diabetes status at baseline revealed similar significant drops in SBP Caution: The Symplicity™ Renal Denervation System is an Investigational Device. Limited by U. S. law to investigational use. Trademarks may be registered and are the property of their respective owners. For OMA distribution only. © 2014 Medtronic, Inc. All rights reserved. 10132474 DOC 02/2014
 Es in research
Es in research Pooled variance estimate formula
Pooled variance estimate formula Pooled variance estimate formula
Pooled variance estimate formula Pooled variance estimate formula
Pooled variance estimate formula Pooled interdependence
Pooled interdependence Pooled standard deviation
Pooled standard deviation Contoh data pooling
Contoh data pooling پلاكت single donor
پلاكت single donor Florida board member certification course
Florida board member certification course Contoh teknologi craft
Contoh teknologi craft Subjects predicates
Subjects predicates Pooled time series cross-section analysis
Pooled time series cross-section analysis