Phy 102 Fundamentals of Physics II Chapter 14



- Slides: 8

Phy 102: Fundamentals of Physics II Chapter 14 Lecture Notes

Robert Boyle (1627 -1691) • Irish-born, English Chemist • The first prominent scientist to carry out controlled experiments – Used rigorous experimental and quantitative methods – Published results in detail • Main Scientific Contributions: – Proposed the first modern definition of a chemical element • Ironically he did not believe in the physical reality of atoms – Designed the first vacuum pump – The first to use color indicators to test acidity • Considered by many to be the “Father of Modern Chemistry”

Gases • Matter that has no definite shape and no definite volume – Take the shape of their container – Take the volume of the container • Gases are fluids • Gas particles have very little interaction with each other (except collisions) • Since gases take the volume of their container, the density is not constant – Depends on temperature and pressure

Atmospheric Pressure • Varies with elevation – decreases as you increase in elevation – decreases as air density decreases Note: Air Pressure = weight density x depth or P=rx. H H is measured from the top down (it is depth not height) However: – Air density (r) decreases as you increase in elevation – At sea level, 1 cubic meter has a mass of 1 -and-¼ kilograms (rair ~ 12. 5 N/m 3)

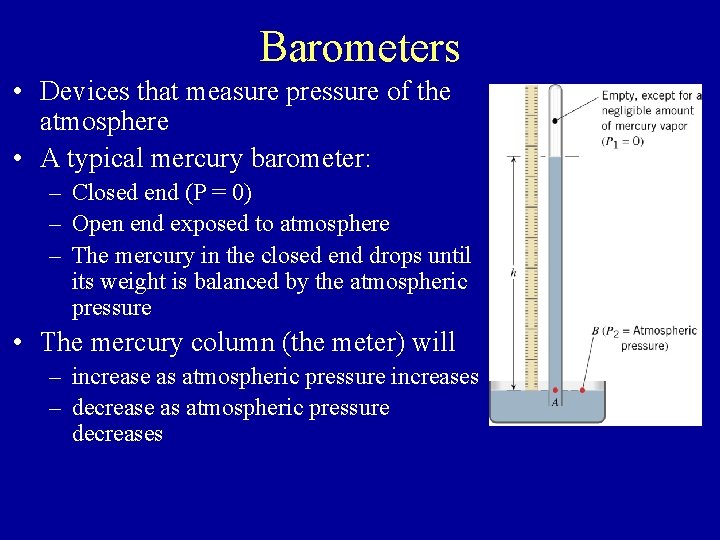
Barometers • Devices that measure pressure of the atmosphere • A typical mercury barometer: – Closed end (P = 0) – Open end exposed to atmosphere – The mercury in the closed end drops until its weight is balanced by the atmospheric pressure • The mercury column (the meter) will – increase as atmospheric pressure increases – decrease as atmospheric pressure decreases

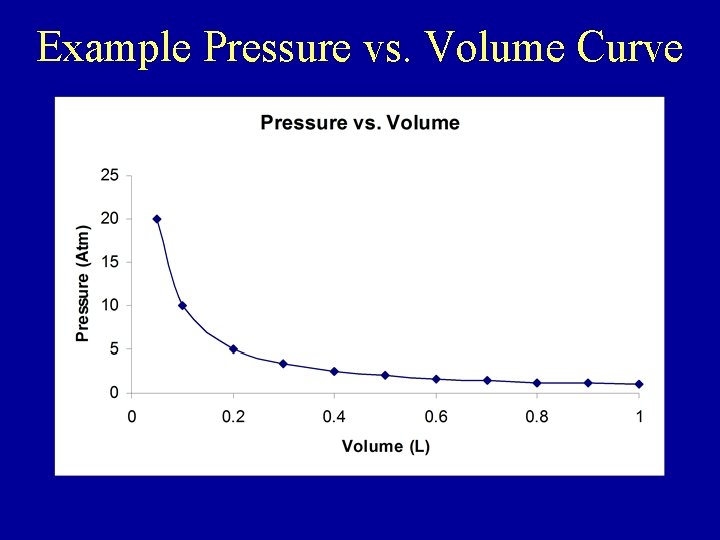
Boyle’s Law • The relationship between pressure & volume for an enclosed gas at constant temperature • Presented by Boyle (but not discovered by him!) • For an enclosed gas the product of pressure times volume is a constant value, or Pressure 1 x Volume 1 = Pressure 2 x Volume 2 or P 1 V 1= P 2 V 2 • Increases/decreases in volume result in inversely proportional change in pressure (vice versa) • Valid only when the temperature is kept constant.

Example Pressure vs. Volume Curve

Bernoulli’s Principle • When the speed of a fluid increases its pressure decreases • Ever ask yourself: – Why do golf balls have dimples? – Why does a pitcher scuff the baseball before throwing it? – Why do loose papers fly out the window when you are driving down the road? – Why don’t airplanes fall out of the sky (at least not most of the time…)? • The answers to these questions, my friends, lie in Bernoulli’s Principle… Pressure Difference = DP = ½ Dv 2