Pearson Prentice Hall Physical Science Concepts in Action















- Slides: 21

Pearson Prentice Hall Physical Science: Concepts in Action Chapter 6 Chemical Bonds

6. 1 Ionic Bonding • Objectives: • 1. Explain when an atom is unlikely to react • 2. Indicate one way in which elements can achieve a stable electron configuration • 3. Express how the structure of an ionic compound affects its properties

When Atoms are Unlikely to React • When the highest occupied energy level (the outer shell) is filled with electrons (valence electrons), the atom is stable and unlikely to react • The valence electrons can be represented by a special drawing called an electron dot diagram

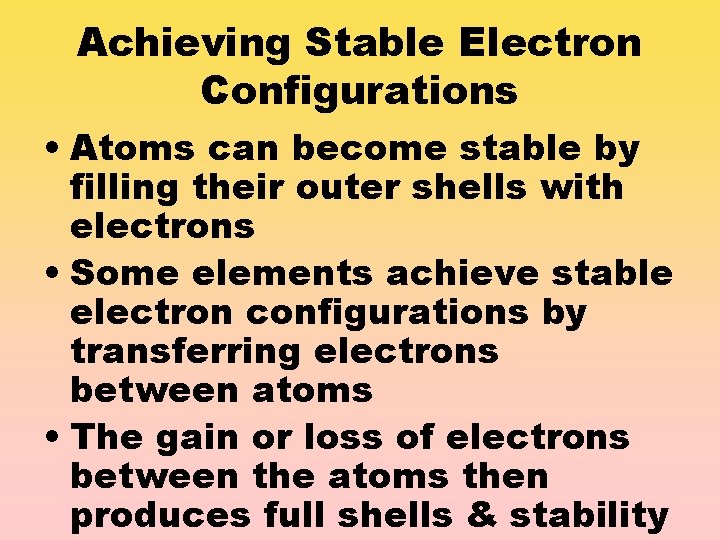
Achieving Stable Electron Configurations • Atoms can become stable by filling their outer shells with electrons • Some elements achieve stable electron configurations by transferring electrons between atoms • The gain or loss of electrons between the atoms then produces full shells & stability

• The gain or loss of electrons also produces an atom with a net positive charge (lost one or more electrons) or a net negative charge (gained one or more electrons) • Definition: an ion is an atom with a net positive or net negative charge • Definition: an anion is an ion with a net negative charge • Definition: a cation is an ion with a net positive charge

• Definition: a chemical bond is the force that holds atoms or ions together as a unit • Definition: an ionic bond is the force that holds cations and anions together • Definition: an ionic bond is the bond that forms when electrons are transferred from one atom to another • Definition: a chemical formula is a notation that shows which elements a compound contains & the ratio of atoms or ions of the elements that make up the compound

• Cations form when electrons gain enough energy to escape from atoms • Electrons must overcome the natural attraction to the protons in the nucleus • Definition: ionization energy is the amount of energy used to remove an electron from an atom • The lower the ionization energy is, the easier it is to remove an electron • Ionization generally increases in the periodic table from left to right and

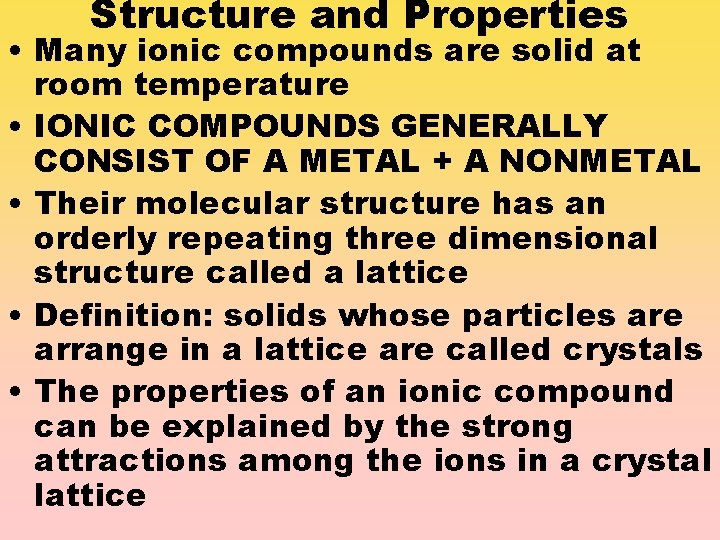
Structure and Properties • Many ionic compounds are solid at room temperature • IONIC COMPOUNDS GENERALLY CONSIST OF A METAL + A NONMETAL • Their molecular structure has an orderly repeating three dimensional structure called a lattice • Definition: solids whose particles are arrange in a lattice are called crystals • The properties of an ionic compound can be explained by the strong attractions among the ions in a crystal lattice

6. 2 Covalent Bonding • Objectives: • 1. Explain how atoms are held together in a covalent bond • 2. Describe what happens when atoms don’t share electrons equally • 3. Discuss what factors determine whether a molecule is polar • 4. Compare the attractions between polar molecules with the attractions between nonpolar molecules

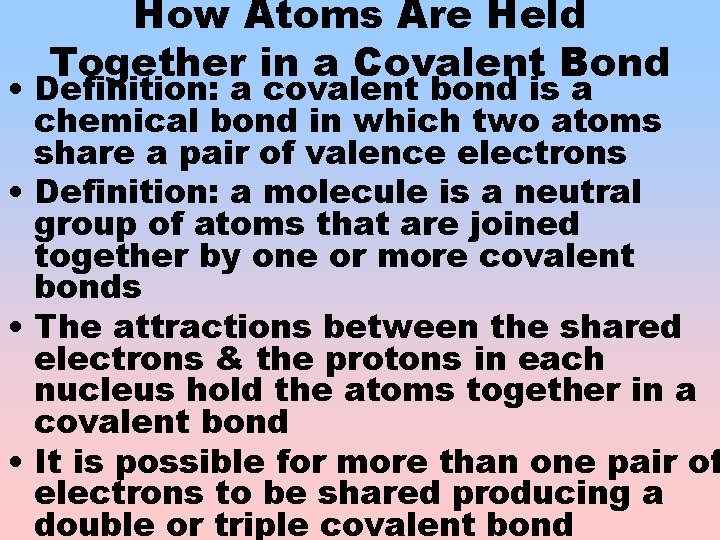
How Atoms Are Held Together in a Covalent Bond • Definition: a covalent bond is a chemical bond in which two atoms share a pair of valence electrons • Definition: a molecule is a neutral group of atoms that are joined together by one or more covalent bonds • The attractions between the shared electrons & the protons in each nucleus hold the atoms together in a covalent bond • It is possible for more than one pair of electrons to be shared producing a double or triple covalent bond

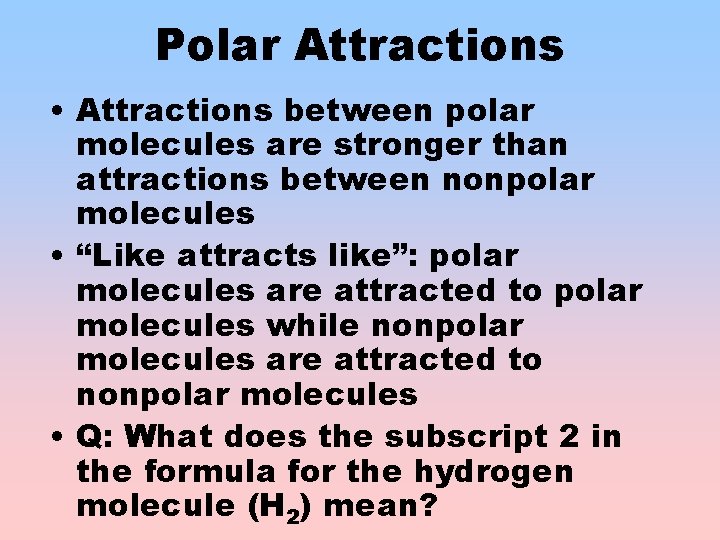
Polar Attractions • Attractions between polar molecules are stronger than attractions between nonpolar molecules • “Like attracts like”: polar molecules are attracted to polar molecules while nonpolar molecules are attracted to nonpolar molecules • Q: What does the subscript 2 in the formula for the hydrogen molecule (H 2) mean?

Unequal Sharing of Electrons • IN GENRAL, COVALENT MOLECULES CONSIST OF TWO NONMETALS • Definition: a polar covalent bond is a covalent bond in which the electrons are not shared equally • When atoms form a polar covalent bond, the atom with the greater attraction for electrons has a partial negative charge (written δ-) • The other atom has a partial positive charge (written δ+) • Water is an example

Factors that Determine Polarity • The type of atoms in a molecule and its geometric shape are factors that determine whether a molecule is polar or nonpolar • Example: CO 2 is linear, while the water molecule is bent

6. 3 Naming Compounds & Writing Formulas • Objectives: • 1. Explain what information the name and formula of an ionic compound provide • 2. Describe the information that the name and formula of a molecular compound provide

Ionic Compound Formulas • The name of an ionic compound (usually a metal plus a nonmetal) must distinguish the compound from other ionic compounds containing the same elements • The formula of an ionic compound describes the ratio of the ions in the compound

• Definition: a binary compound is a compound made of only 2 elements • The name of a binary compound follows a pattern • The cation (+ charge, usually the metal) is named first followed by the anion (- charge, usually the nonmetal) • The cation states its name • There is a special suffix for the anion • The anion uses the stem of its name plus –ide ex: chlorine becomes chloride (-ide means it’s an ion)

• Many transition metals can form more than one ion • Ex: iron can be Fe 2+ or Fe 3+ & is called “iron two ion” or “iron three ion” • The name of transition metal ions will have a Roman numeral after the name that states the charge • Ex: Cu. O is copper(II) oxide because copper can be Cu+ or Cu 2+ • Definition: a polyatomic ion is a covalently bonded group of atoms acting as a single unit that carries a charge • Compounds with polyatomic ions are ionic compounds

• If you know the name, you can write the formula • The total charges of the cations and anions must add up to zero • Sodium ion is Na+ while sulfide is S 2 • You will need 2 sodium ions to make the charges add up to zero • It is written Na 2 S and is called sodium sulfide

Molecular Compound Formulas • The name and formula of a molecular compound describe the type and number of atoms in a molecule of that compound • Molecular compounds are made from nonmetals (upstairs to the right in the periodic table) • To name the compounds, the most metallic element, farthest to the left on the table is named first • If the elements are in the same group, the element closest to the bottom of the group is named first

• The suffix of the second element is changed to –ide • It is possible to for 2 elements to combine in different amounts to produce a molecular compound such as N 2 O 4 and NO 2 • Prefixes are used to tell the amount of each except when there is only one atom of the first element • Do not use –mono on the first element only • 1=mono- 2=di- 3=tri- 4=tetra 5=penta- 6=hexa- 7=hepta- 8=octa 9=nona- 10=deca-

• To write the formula for a molecular compound, write the symbols of the elements in the name • Then add the appropriate subscript to the element • Ex: diphosphorus tetrafluoride is P 2 F 4 • Q: What is a polyatomic ion? • Q: What is the formula for calcium chloride? • Q: Is calcium chloride an ionic or molecular compound? How do you know?