Olesya A Krumkacheva Vitaly R Gorelik Elena G




- Slides: 14

Olesya A. Krumkacheva†, Vitaly R. Gorelik†, Elena G. Bagryanskaya†, Natalia V. Lebedeva‡ and Malcolm D. E. Forbes‡ † International Tomography Center, Institutskaya 3 a, Novosibirsk 630090, Russia Caudill Laboratories, Department of Chemistry, CB #3290, University of North Carolina, Chapel Hill, North Carolina 27599 -3290 ‡ Supramolecular Photochemistry in β-Cyclodextrin Hosts: A TREPR, NMR, and CIDNP Investigation

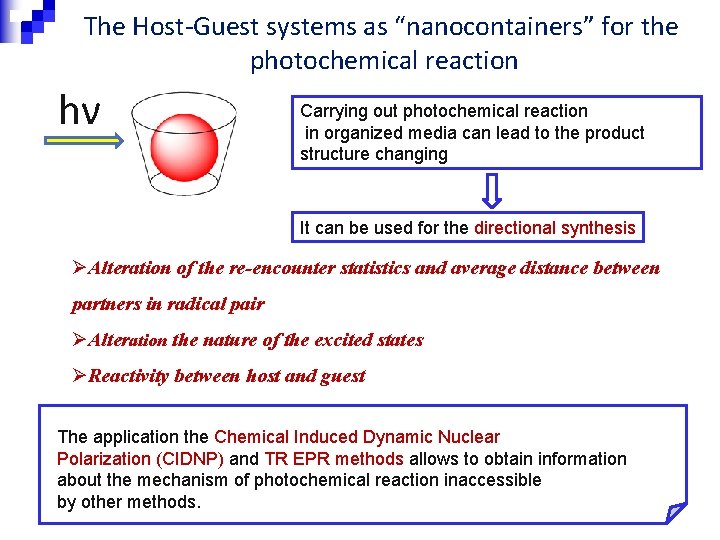
The Host-Guest systems as “nanocontainers” for the photochemical reaction hν Carrying out photochemical reaction in organized media can lead to the product structure changing It can be used for the directional synthesis ØAlteration of the re-encounter statistics and average distance between partners in radical pair ØAlteration the nature of the excited states ØReactivity between host and guest The application the Chemical Induced Dynamic Nuclear Polarization (CIDNP) and TR EPR methods allows to obtain information about the mechanism of photochemical reaction inaccessible by other methods.

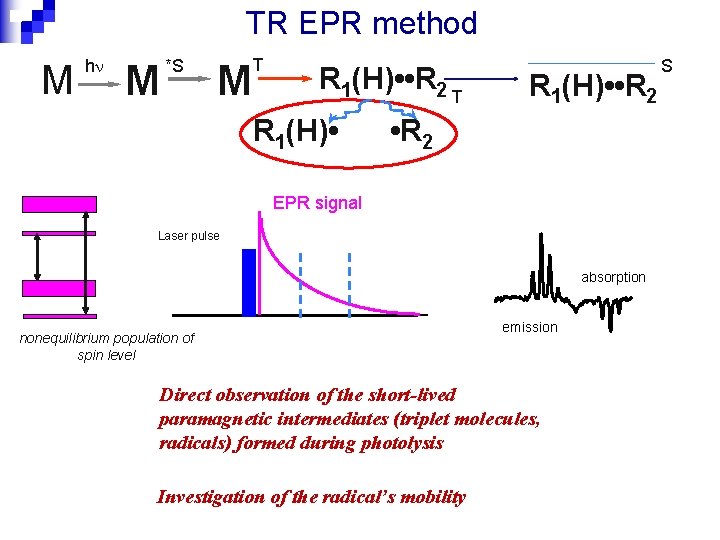
TR EPR method M h M *S T M R 1(H) • • R 2 T R 1(H) • • R 2 EPR signal Laser pulse absorption nonequilibrium population of spin level emission Direct observation of the short-lived paramagnetic intermediates (triplet molecules, radicals) formed during photolysis Investigation of the radical’s mobility S

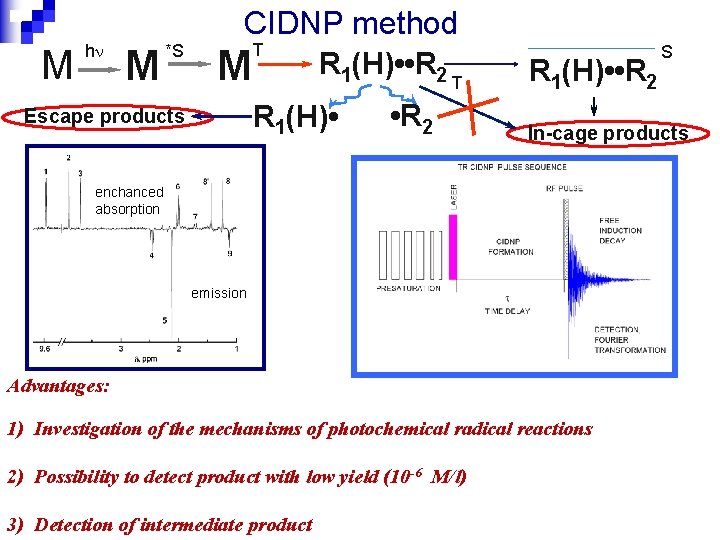
CIDNP method M h M *S T M R 1(H) • • R 2 T R 1(H) • Escape products • R 2 R 1(H) • • R 2 In-cage products enchanced absorption emission Advantages: 1) Investigation of the mechanisms of photochemical radical reactions 2) Possibility to detect product with low yield (10 -6 M/l) 3) Detection of intermediate product S

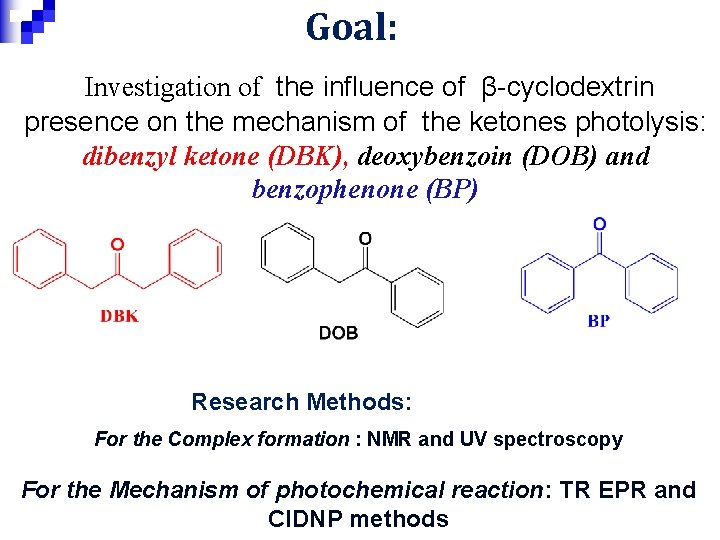
Goal: Investigation of the influence of β-cyclodextrin presence on the mechanism of the ketones photolysis: dibenzyl ketone (DBK), deoxybenzoin (DOB) and benzophenone (BP) Research Methods: For the Complex formation : NMR and UV spectroscopy For the Mechanism of photochemical reaction: TR EPR and CIDNP methods

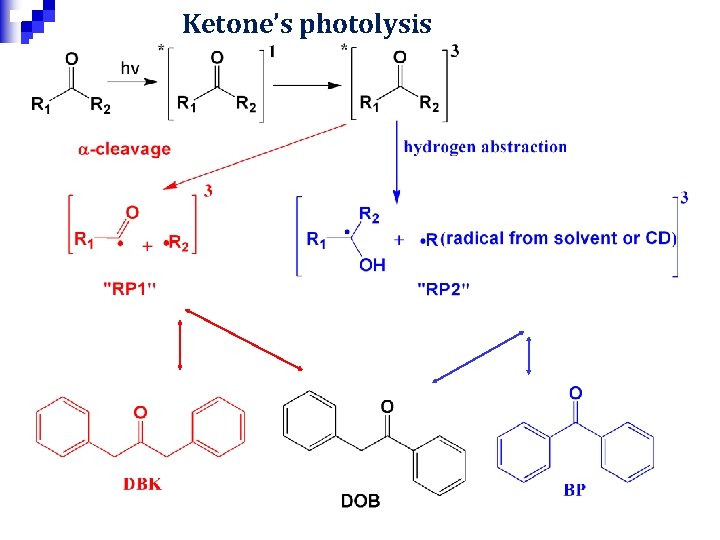
Ketone’s photolysis

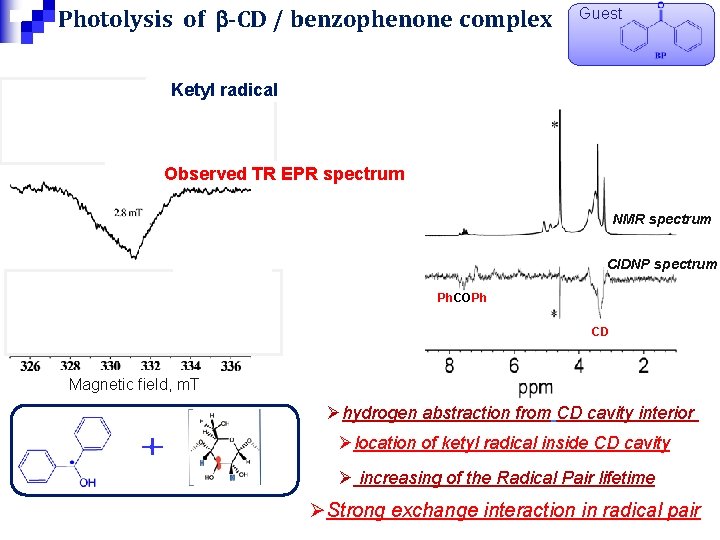
Photolysis of b-CD / benzophenone complex Guest Ketyl radical Observed TR EPR spectrum NMR spectrum CD radical CIDNP spectrum Ph. COPh CD Magnetic field, m. T Øhydrogen abstraction from CD cavity interior Ølocation of ketyl radical inside CD cavity Ø increasing of the Radical Pair lifetime ØStrong exchange interaction in radical pair

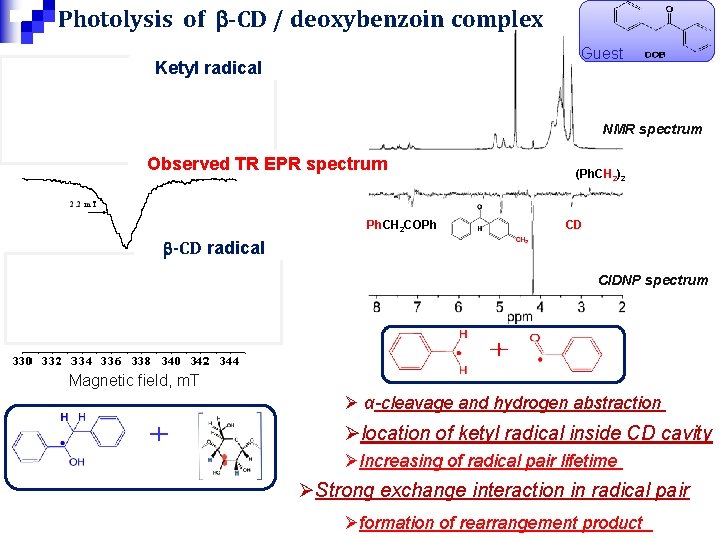
Photolysis of b-CD / deoxybenzoin complex Guest Ketyl radical NMR spectrum Observed TR EPR spectrum Ph. CH 2 COPh (Ph. CH 2)2 CD b-CD radical CIDNP spectrum Magnetic field, m. T Ø α-cleavage and hydrogen abstraction Ølocation of ketyl radical inside CD cavity ØIncreasing of radical pair lifetime ØStrong exchange interaction in radical pair Øformation of rearrangement product

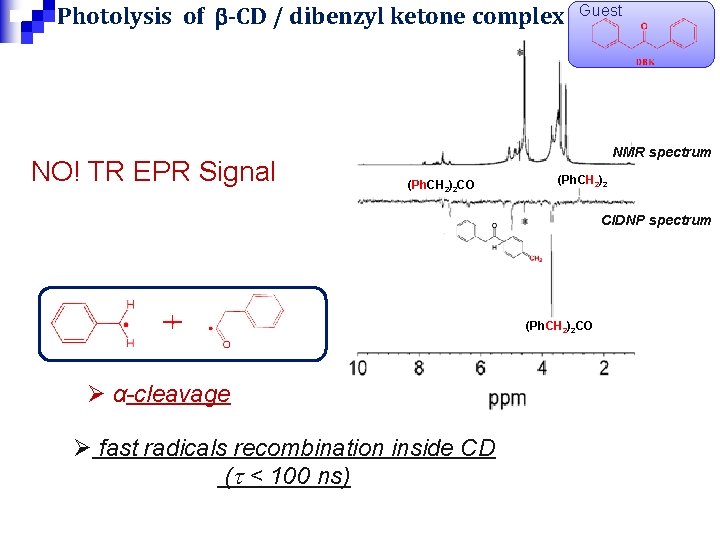
Photolysis of b-CD / dibenzyl ketone complex NO! TR EPR Signal Guest NMR spectrum (Ph. CH 2)2 CO (Ph. CH 2)2 CIDNP spectrum (Ph. CH 2)2 CO Ø α-cleavage Ø fast radicals recombination inside CD (t < 100 ns)

Results ØPhotophysics and photochemistry of DOB, DBK, and BP in βCD inclusion complexes had been examined in detail. The DOB triplet state undergoes both reactions whereas the DBK triplet shows exclusively a-cleavage and the BP triplet shows exclusively H-atom abstraction. ØObservation of rearrangement product of the radicals from acleavage implies that there is substantial mobility of the radicals into the CD interior ØIt was found that there is a fast radicals recombination inside CD in the case of the a-cleavage reaction (t < 100 ns) ØIt was shown that there is a strong exchange interaction between the ketyl and CD radicals, due to location of ketyl radical inside CD. Langmuir, 2010, 26 (11), pp 8971– 8980

Supramolecular Photochemistry in β-Cyclodextrin Hosts: A TREPR, NMR, and CIDNP Investigation Olesya A. Krumkacheva International Tomography Center SB RAS olesya@tomo. nsc. ru Thank you for your kind attention !

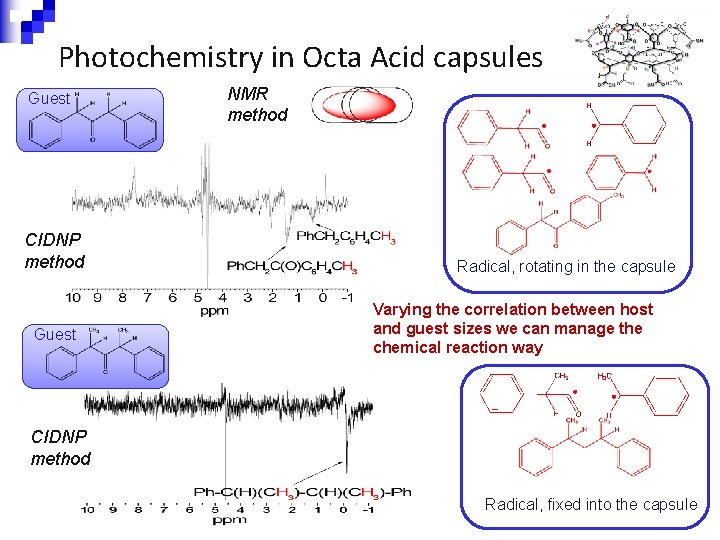
Photochemistry in Octa Acid capsules Guest CIDNP method Guest NMR method Radical, rotating in the capsule Varying the correlation between host and guest sizes we can manage the chemical reaction way CIDNP method Radical, fixed into the capsule