NRTIsparing SPARTAN PROGRESS RADAR NEAT 001ANRS 143 A

- Slides: 4

NRTI-sparing § § § § SPARTAN PROGRESS RADAR NEAT 001/ANRS 143 A 4001078 VEMAN MODERN

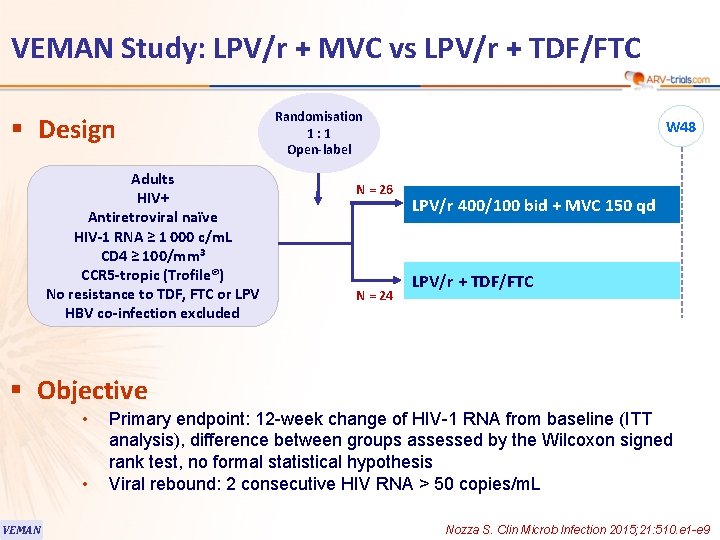
118 VEMAN Study: LPV/r + MVC vs LPV/r + TDF/FTC § Design Adults HIV+ Antiretroviral naïve HIV-1 RNA ≥ 1 000 c/m. L CD 4 ≥ 100/mm 3 CCR 5 -tropic (Trofile®) No resistance to TDF, FTC or LPV HBV co-infection excluded Randomisation 1: 1 Open-label N = 26 N = 24 W 48 LPV/r 400/100 bid + MVC 150 qd LPV/r + TDF/FTC § Objective • • VEMAN Primary endpoint: 12 -week change of HIV-1 RNA from baseline (ITT analysis), difference between groups assessed by the Wilcoxon signed rank test, no formal statistical hypothesis Viral rebound: 2 consecutive HIV RNA > 50 copies/m. L Nozza S. Clin Microb Infection 2015; 21: 510. e 1 -e 9

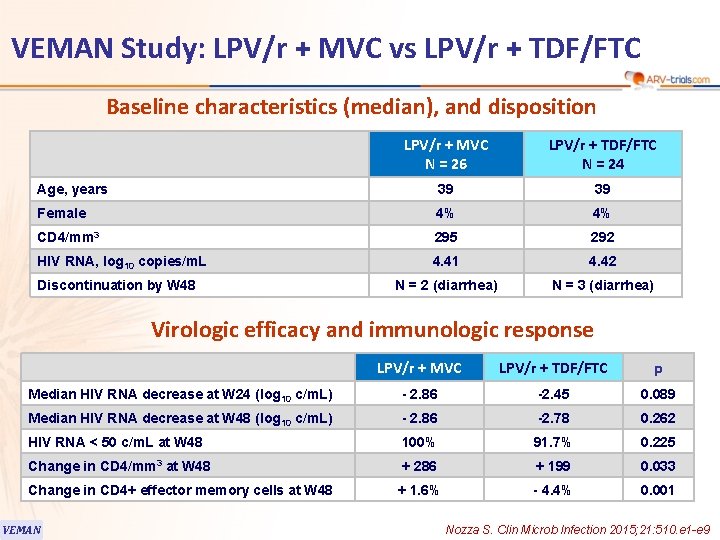
VEMAN Study: LPV/r + MVC vs LPV/r + TDF/FTC Baseline characteristics (median), and disposition LPV/r + MVC N = 26 LPV/r + TDF/FTC N = 24 Age, years 39 39 Female 4% 4% CD 4/mm 3 295 292 HIV RNA, log 10 copies/m. L 4. 41 4. 42 N = 2 (diarrhea) N = 3 (diarrhea) Discontinuation by W 48 Virologic efficacy and immunologic response LPV/r + MVC LPV/r + TDF/FTC p Median HIV RNA decrease at W 24 (log 10 c/m. L) - 2. 86 -2. 45 0. 089 Median HIV RNA decrease at W 48 (log 10 c/m. L) - 2. 86 -2. 78 0. 262 HIV RNA < 50 c/m. L at W 48 100% 91. 7% 0. 225 Change in CD 4/mm 3 at W 48 + 286 + 199 0. 033 + 1. 6% - 4. 4% 0. 001 Change in CD 4+ effector memory cells at W 48 VEMAN Nozza S. Clin Microb Infection 2015; 21: 510. e 1 -e 9

VEMAN Study: LPV/r + MVC vs LPV/r + TDF/FTC § Conclusion – Among naive patients with an R 5 virus, treatment with MVC and LPV/r was shown to provide a virological response compared to a triple therapy and a greater immunological benefit – Limitations • Small sample size • Unpowered to establish non-inferiority • AEs self-reported, open-label unblinded design VEMAN Nozza S. Clin Microb Infection 2015; 21: 510. e 1 -e 9







