Moscow Healthcare Department Moscow clinical hospital 52 management


- Slides: 10

ДЕПАРТАМЕНТ ЗДРАВООХРАНЕНИЯ ГОРОДА МОСКВЫ Moscow Healthcare Department Moscow clinical hospital № 52: management of patient with community-acquired pneumonia (CAP) suspected COVID and COVID +

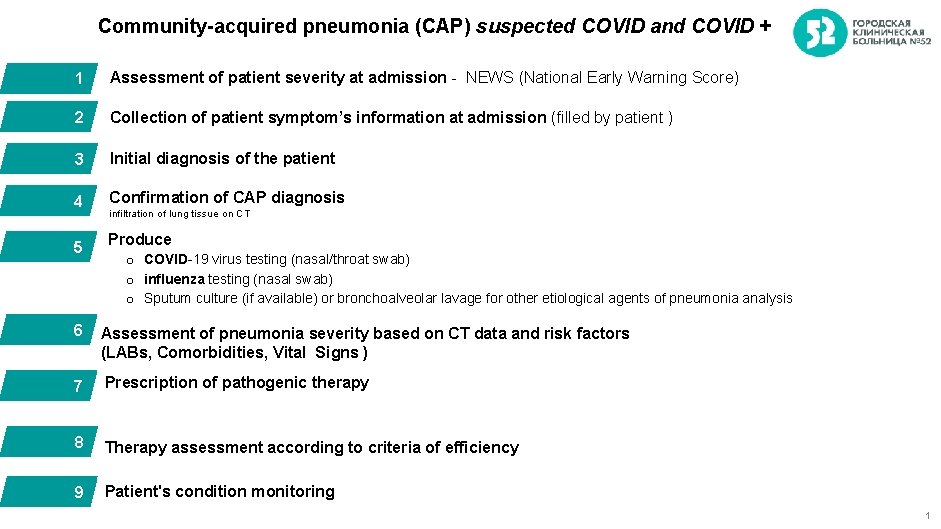
Community-acquired pneumonia (CAP) suspected COVID and COVID + 1 Assessment of patient severity at admission - NEWS (National Early Warning Score) 2 Collection of patient symptom’s information at admission (filled by patient ) 3 Initial diagnosis of the patient 4 Confirmation of CAP diagnosis 5 Produce infiltration of lung tissue on CT o COVID-19 virus testing (nasal/throat swab) o influenza testing (nasal swab) o Sputum culture (if available) or bronchoalveolar lavage for other etiological agents of pneumonia analysis 6 Assessment of pneumonia severity based on CT data and risk factors (LABs, Comorbidities, Vital Signs ) 7 Prescription of pathogenic therapy 8 Therapy assessment according to criteria of efficiency 9 Patient's condition monitoring 1

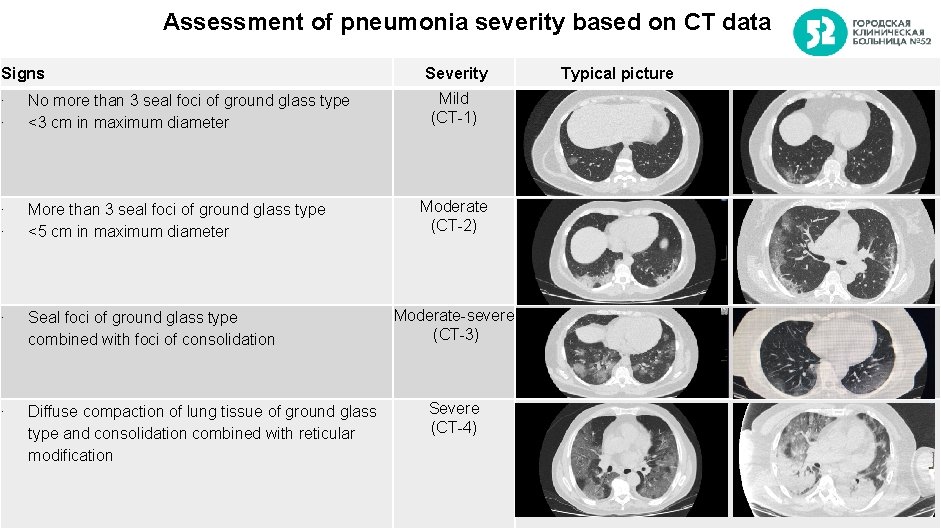
Assessment of pneumonia severity based on CT data Signs ∙ ∙ No more than 3 seal foci of ground glass type <3 cm in maximum diameter ∙ ∙ More than 3 seal foci of ground glass type <5 cm in maximum diameter ∙ Seal foci of ground glass type combined with foci of consolidation ∙ Diffuse compaction of lung tissue of ground glass type and consolidation combined with reticular modification Severity Typical picture Mild (CT-1) Moderate (CT-2) Moderate-severe (СT-3) Severe (CT-4) 2

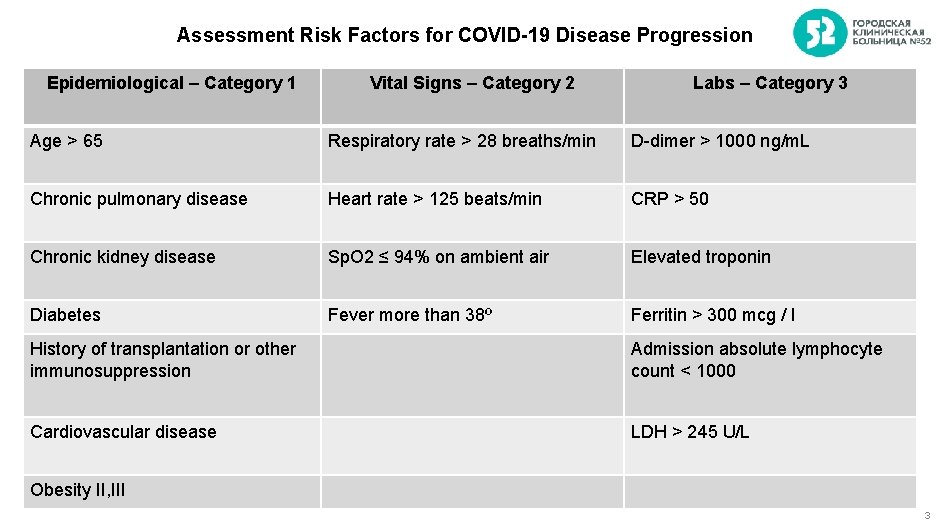
Assessment Risk Factors for COVID-19 Disease Progression Epidemiological – Category 1 Vital Signs – Category 2 Labs – Category 3 Age > 65 Respiratory rate > 28 breaths/min D-dimer > 1000 ng/m. L Chronic pulmonary disease Heart rate > 125 beats/min CRP > 50 Chronic kidney disease Sp. O 2 ≤ 94% on ambient air Elevated troponin Diabetes Fever more than 38º Ferritin > 300 mcg / l History of transplantation or other immunosuppression Admission absolute lymphocyte count < 1000 Cardiovascular disease LDH > 245 U/L Obesity II, III 3

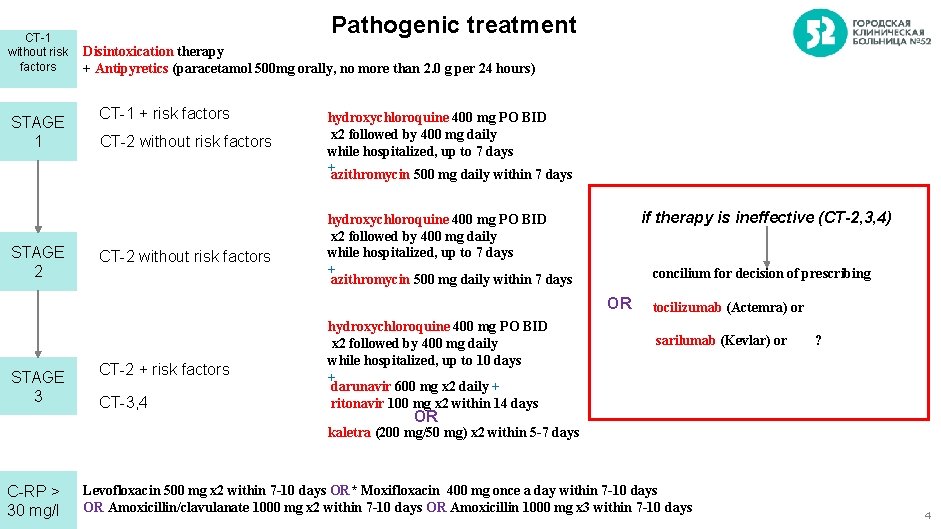
CT-1 without risk factors STAGE 1 STAGE 2 Pathogenic treatment Disintoxication therapy + Antipyretics (paracetamol 500 mg orally, no more than 2. 0 g per 24 hours) СT-1 + risk factors СT-2 without risk factors hydroxychloroquine 400 mg PO BID x 2 followed by 400 mg daily while hospitalized, up to 7 days +azithromycin 500 mg daily within 7 days if therapy is ineffective (CT-2, 3, 4) hydroxychloroquine 400 mg PO BID x 2 followed by 400 mg daily while hospitalized, up to 7 days + azithromycin 500 mg daily within 7 days concilium for decision of prescribing OR STAGE 3 СТ-2 + risk factors СT-3, 4 hydroxychloroquine 400 mg PO BID x 2 followed by 400 mg daily while hospitalized, up to 10 days + darunavir 600 mg x 2 daily + ritonavir 100 mg x 2 within 14 days tocilizumab (Actemra) or sarilumab (Kevlar) or ? OR kaletra (200 mg/50 mg) x 2 within 5 -7 days C-RP > 30 mg/l Levofloxacin 500 mg x 2 within 7 -10 days OR* Moxifloxacin 400 mg once a day within 7 -10 days OR Amoxicillin/clavulanate 1000 mg x 2 within 7 -10 days OR Amoxicillin 1000 mg x 3 within 7 -10 days 4

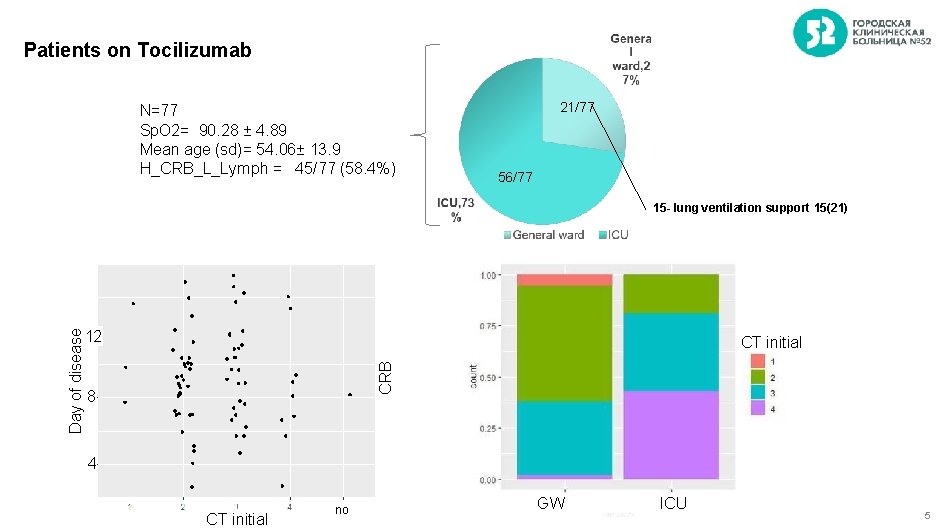
Patients on Tocilizumab N=77 Sp. O 2= 90. 28 ± 4. 89 Mean age (sd)= 54. 06± 13. 9 H_CRB_L_Lymph = 45/77 (58. 4%) 21/77 56/77 15 - lung ventilation support 15(21) CT initial CRB Day of disease 12 8 4 CT initial no GW ICU 5

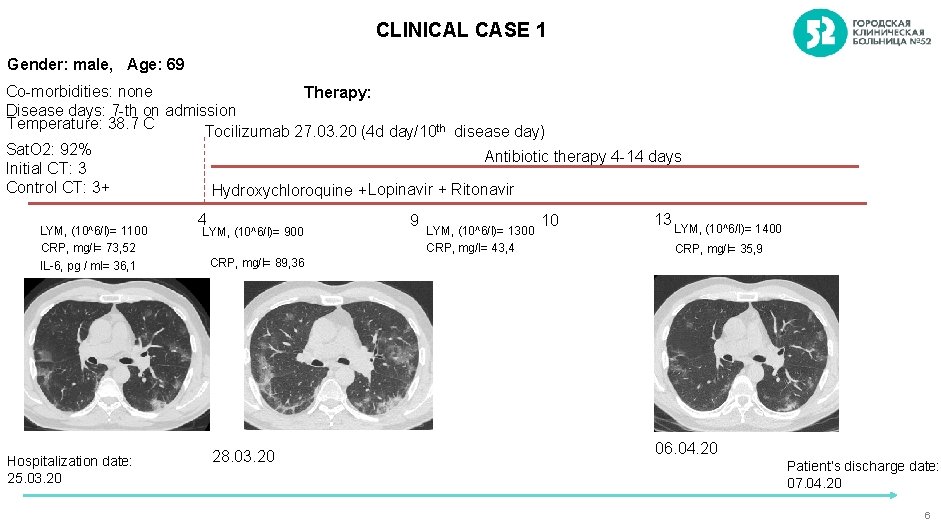
CLINICAL CASE 1 Gender: male, Age: 69 Co-morbidities: none Therapy: Disease days: 7 -th on admission Temperature: 38. 7 C Tocilizumab 27. 03. 20 (4 d day/10 th disease day) Sat. O 2: 92% Antibiotic therapy 4 -14 days Initial CT: 3 Control CT: 3+ Hydroxychloroquine + Lopinavir + Ritonavir LYM, (10^6/l)= 1100 CRP, mg/l= 73, 52 IL-6, pg / ml= 36, 1 Hospitalization date: 25. 03. 20 4 LYM, (10^6/l)= 900 9 LYM, (10^6/l)= 1300 CRP, mg/l= 43, 4 10 13 LYM, (10^6/l)= 1400 CRP, mg/l= 35, 9 CRP, mg/l= 89, 36 28. 03. 20 06. 04. 20 Patient’s discharge date: 07. 04. 20 6

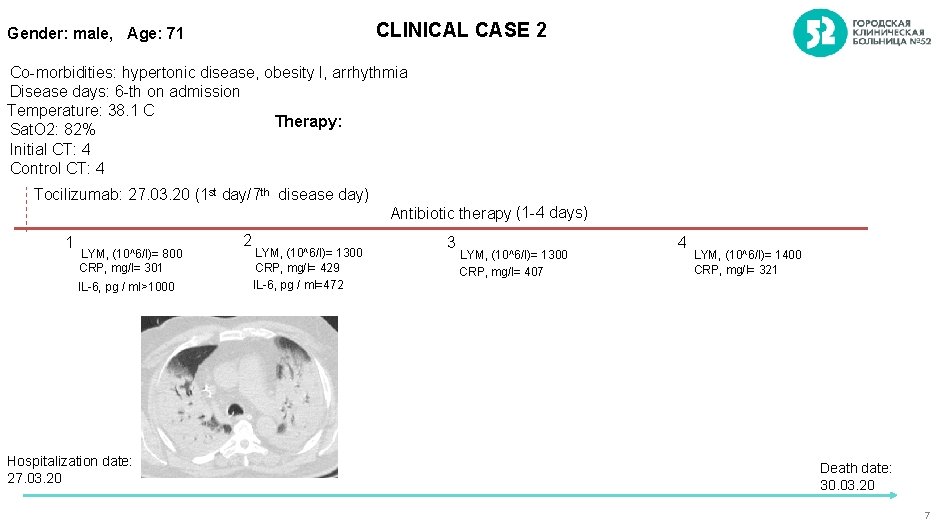
CLINICAL CASE 2 Gender: male, Age: 71 Co-morbidities: hypertonic disease, obesity I, arrhythmia Disease days: 6 -th on admission Temperature: 38. 1 C Therapy: Sat. O 2: 82% Initial CT: 4 Control CT: 4 Tocilizumab: 27. 03. 20 (1 st day/7 th disease day) 1 LYM, (10^6/l)= 800 CRP, mg/l= 301 IL-6, pg / ml>1000 Hospitalization date: 27. 03. 20 2 LYM, (10^6/l)= 1300 CRP, mg/l= 429 IL-6, pg / ml=472 Antibiotic therapy (1 -4 days) 3 LYM, (10^6/l)= 1300 CRP, mg/l= 407 4 LYM, (10^6/l)= 1400 CRP, mg/l= 321 Death date: 30. 03. 20 7

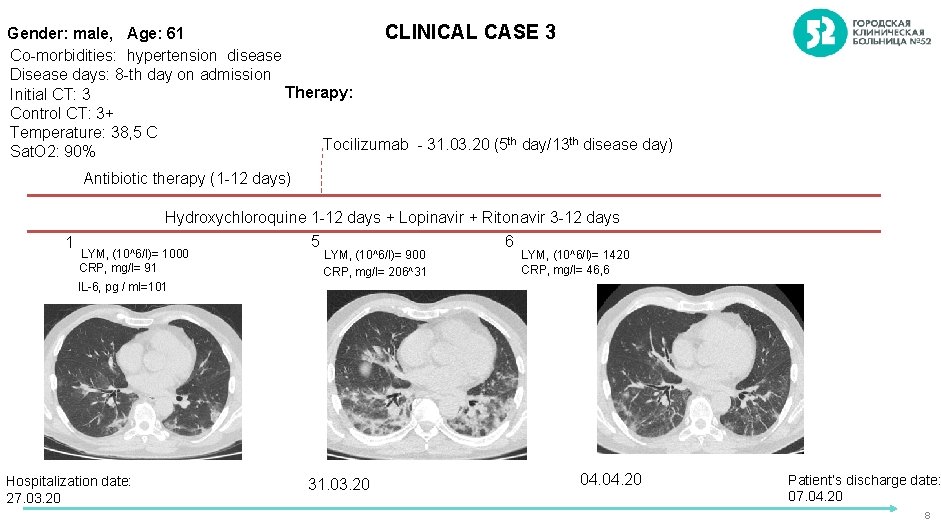
CLINICAL CASE 3 Gender: male, Age: 61 Co-morbidities: hypertension disease Disease days: 8 -th day on admission Therapy: Initial CT: 3 Control CT: 3+ Temperature: 38, 5 C Tocilizumab - 31. 03. 20 (5 th day/13 th disease day) Sat. O 2: 90% Antibiotic therapy (1 -12 days) 1 Hydroxychloroquine 1 -12 days + Lopinavir + Ritonavir 3 -12 days 5 6 LYM, (10^6/l)= 1000 CRP, mg/l= 91 LYM, (10^6/l)= 900 CRP, mg/l= 206^31 LYM, (10^6/l)= 1420 CRP, mg/l= 46, 6 IL-6, pg / ml=101 Hospitalization date: 27. 03. 20 31. 03. 20 04. 20 Patient’s discharge date: 07. 04. 20 8

Thank you for your attention ! 9
 Healthcare and the healthcare team chapter 2
Healthcare and the healthcare team chapter 2 Sports medicine definition
Sports medicine definition Healthcare finance department structure
Healthcare finance department structure Organisation of hospital pharmacy
Organisation of hospital pharmacy Hesf1
Hesf1 Organizational structure of a hospital
Organizational structure of a hospital Flagler hospital billing department
Flagler hospital billing department Value stream management for lean healthcare
Value stream management for lean healthcare Service line strategy
Service line strategy Healthcare industry knowledge
Healthcare industry knowledge Healthcare industry document management system
Healthcare industry document management system



















