Modulating the Antioxidant Defences to Potentiate Oncolytic Virotherapy

- Slides: 18

Modulating the Antioxidant Defences to Potentiate Oncolytic Virotherapy in Refractory Cancer Cells 5 th European Immunology & 2 nd Innate Immunity and Immune System Diseases Berlin, Germany July 22, 2016 & Im diclege September 9, 2015 Rongtuan Lin, Ph. D. Lady Davis Institute/Mc. Gill University Montreal, QC Canada

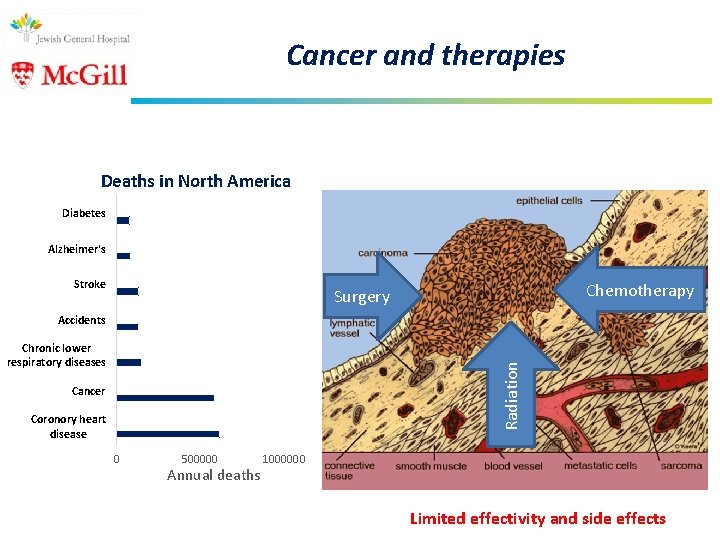
Cancer and therapies Deaths in North America Diabetes Alzheimer's Stroke Chemotherapy Surgery Accidents Radiation Chronic lower respiratory diseases Cancer Coronory heart disease 0 500000 Annual deaths 1000000 Limited effectivity and side effects

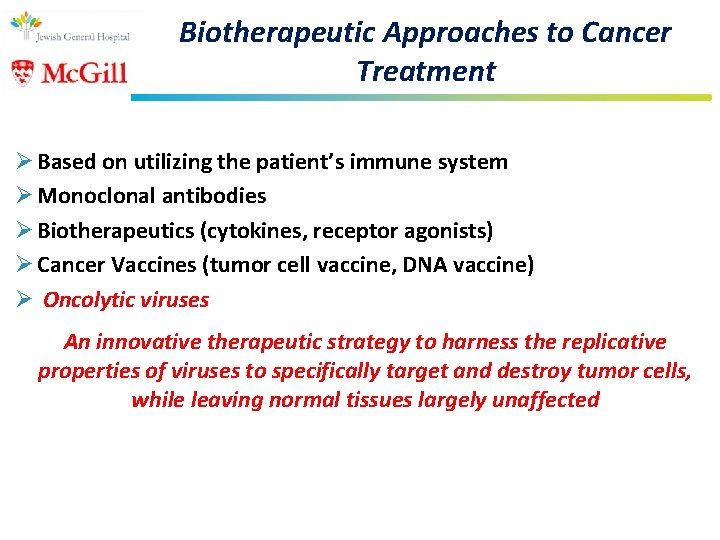
Biotherapeutic Approaches to Cancer Treatment Ø Based on utilizing the patient’s immune system Ø Monoclonal antibodies Ø Biotherapeutics (cytokines, receptor agonists) Ø Cancer Vaccines (tumor cell vaccine, DNA vaccine) Ø Oncolytic viruses An innovative therapeutic strategy to harness the replicative properties of viruses to specifically target and destroy tumor cells, while leaving normal tissues largely unaffected

Oncolytic Viruses in Cancer Treatment • Cancer cells have a dampened innate immune response • They are more susceptible to pathogens • Use viruses to infect and kill cancer cells

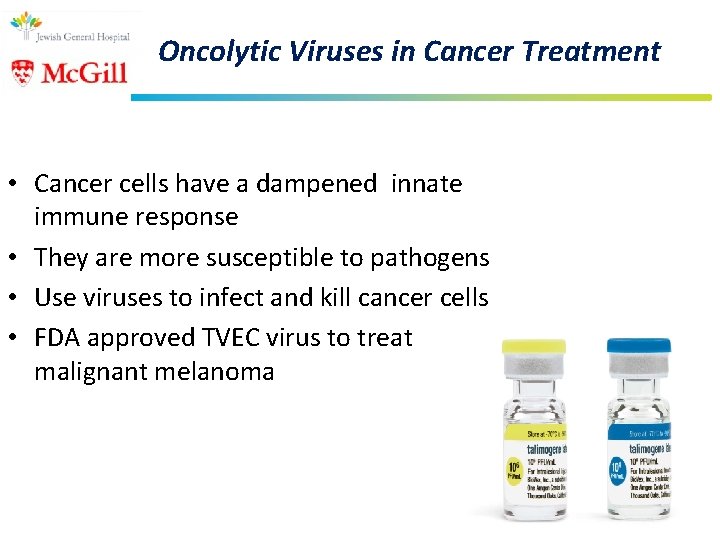
Oncolytic Viruses in Cancer Treatment • Cancer cells have a dampened innate immune response • They are more susceptible to pathogens • Use viruses to infect and kill cancer cells • FDA approved TVEC virus to treat malignant melanoma

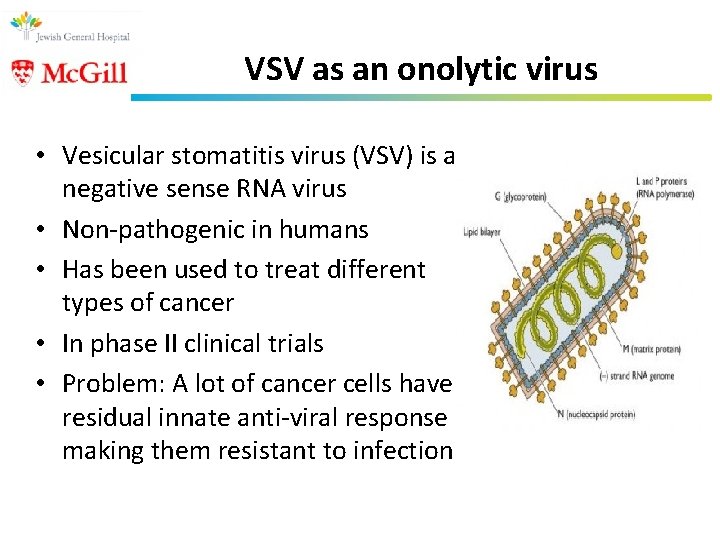
VSV as an onolytic virus • Vesicular stomatitis virus (VSV) is a negative sense RNA virus • Non-pathogenic in humans • Has been used to treat different types of cancer • In phase II clinical trials • Problem: A lot of cancer cells have a residual innate anti-viral response making them resistant to infection

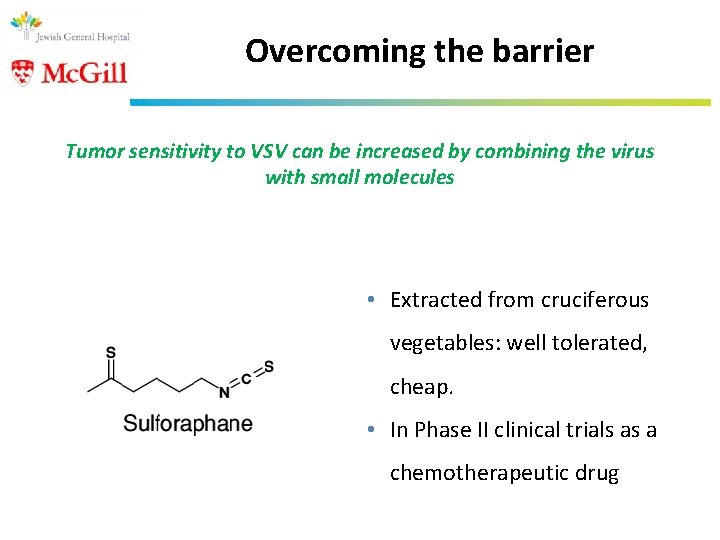
Overcoming the barrier Tumor sensitivity to VSV can be increased by combining the virus with small molecules • Extracted from cruciferous vegetables: well tolerated, cheap. • In Phase II clinical trials as a chemotherapeutic drug

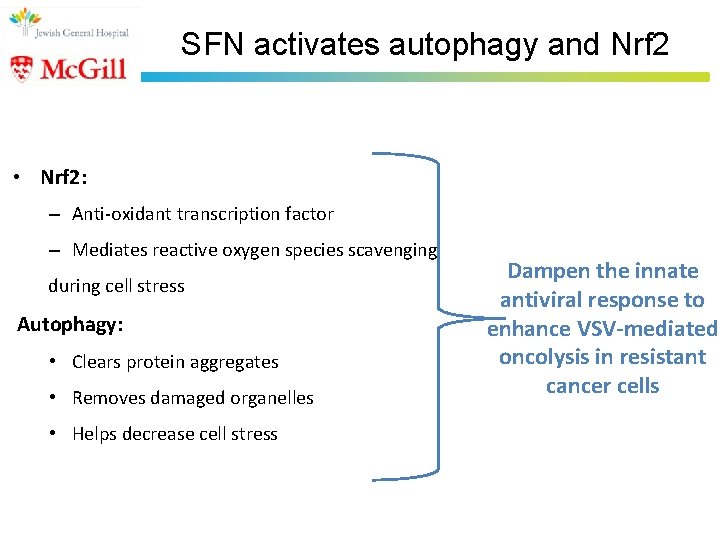
SFN activates autophagy and Nrf 2 • Nrf 2: – Anti-oxidant transcription factor – Mediates reactive oxygen species scavenging during cell stress Autophagy: • Clears protein aggregates • Removes damaged organelles • Helps decrease cell stress Dampen the innate antiviral response to enhance VSV-mediated oncolysis in resistant cancer cells

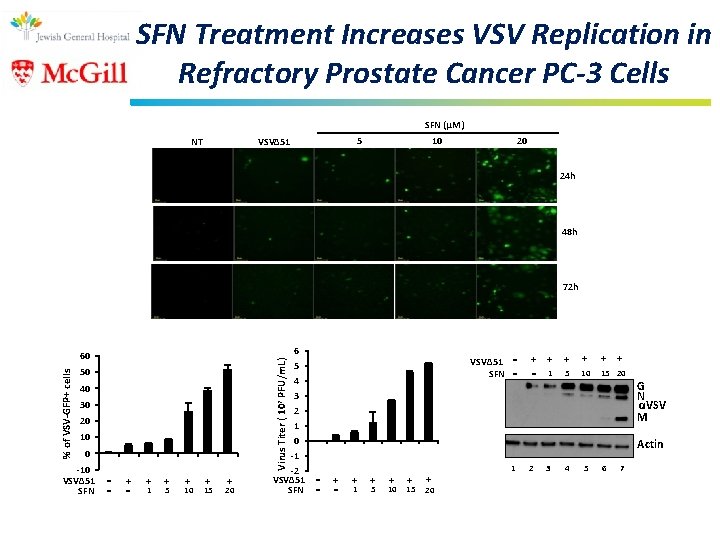
SFN Treatment Increases VSV Replication in Refractory Prostate Cancer PC-3 Cells SFN (μM) 5 VSVΔ 51 NT 10 20 24 h 48 h 72 h 50 40 30 20 10 0 -10 VSVΔ 51 SFN - + - - 6 5 4 3 2 1 0 -1 -2 VSVΔ 51 SFN Virus Titer ( 107 PFU/m. L) % of VSV-GFP+ cells 60 + 1 + 5 + 10 + 15 + 20 VSVΔ 51 SFN - + - - + + + 5 15 20 1 10 G N αVSV M Actin - + - - + 1 + 5 + 10 + 15 + 20 1 2 3 4 5 6 7

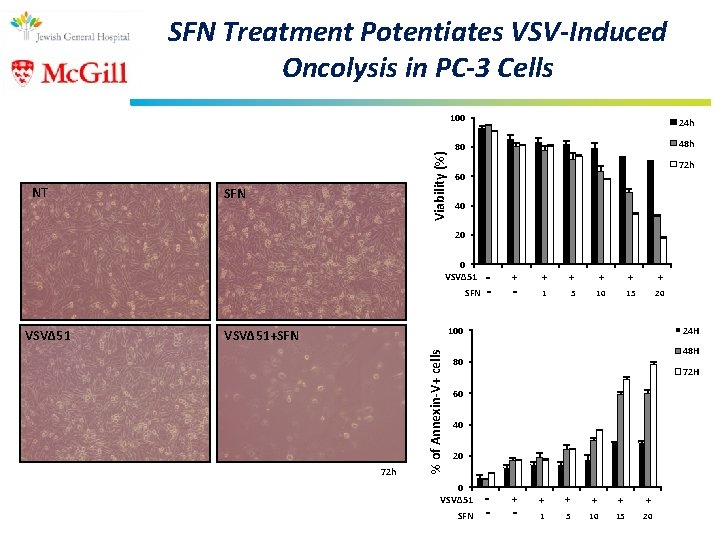
NT Viability (%) SFN Treatment Potentiates VSV-Induced Oncolysis in PC-3 Cells SFN 100 24 h 80 48 h 72 h 60 40 20 0 VSVΔ 51 SFN + - + + + 1 5 10 15 20 24 H 100 VSVΔ 51+SFN 72 h % of Annexin-V+ cells VSVΔ 51 - 48 H 80 72 H 60 40 20 0 VSVΔ 51 SFN - + + + 1 5 10 15 20

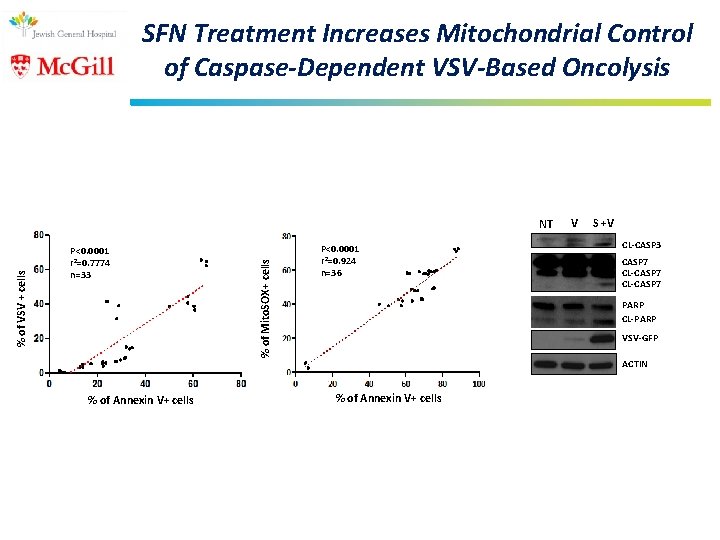
SFN Treatment Increases Mitochondrial Control of Caspase-Dependent VSV-Based Oncolysis P<0. 0001 r 2=0. 7774 n=33 % of Annexin V+ cells % of Mito. SOX+ cells % of VSV + cells NT P<0. 0001 r 2=0. 924 n=36 V S +V CL-CASP 3 CASP 7 CL-CASP 7 PARP CL-PARP VSV-GFP ACTIN % of Annexin V+ cells

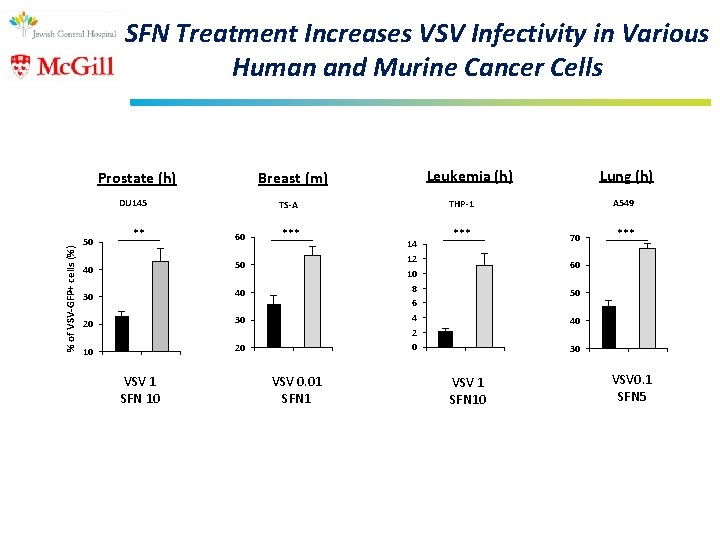
SFN Treatment Increases VSV Infectivity in Various Human and Murine Cancer Cells Prostate (h) % of VSV-GFP+ cells (%) DU 145 50 ** 60 50 30 40 20 30 10 20 *** VSV 0. 01 SFN 1 Lung (h) A 549 THP-1 TS-A 40 VSV 1 SFN 10 Leukemia (h) Breast (m) 14 12 10 8 6 4 2 0 *** 70 *** 60 50 40 30 VSV 1 SFN 10 VSV 0. 1 SFN 5

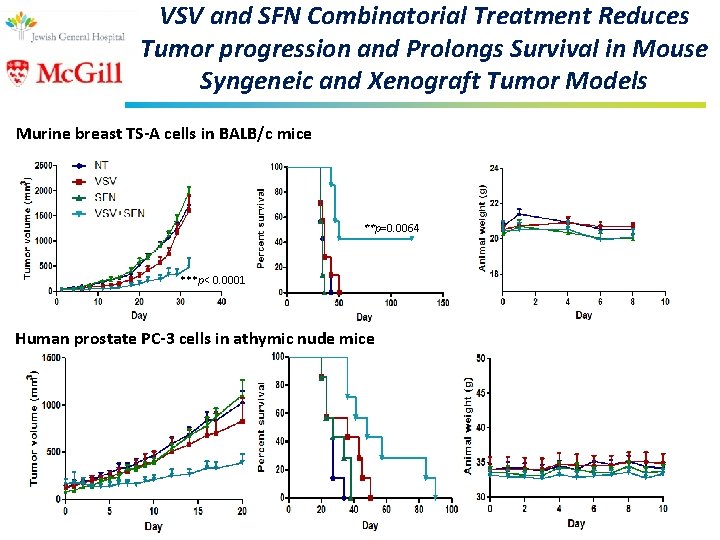
VSV and SFN Combinatorial Treatment Reduces Tumor progression and Prolongs Survival in Mouse Syngeneic and Xenograft Tumor Models Murine breast TS-A cells in BALB/c mice **p=0. 0064 ***p< 0. 0001 Human prostate PC-3 cells in athymic nude mice

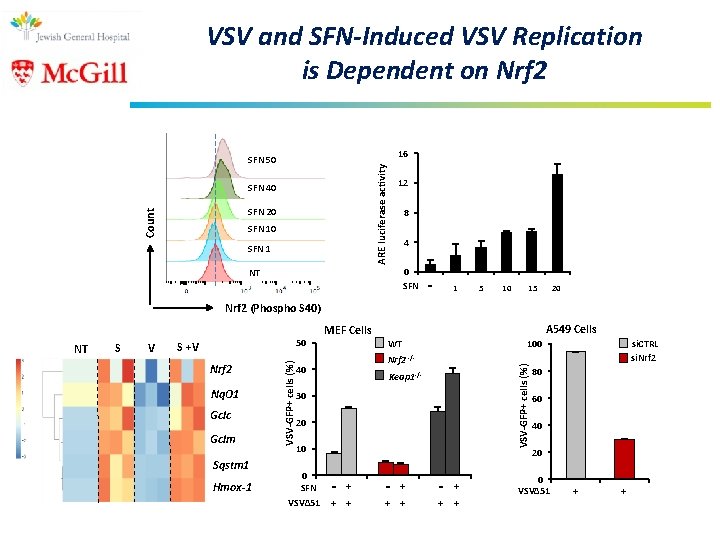
VSV and SFN-Induced VSV Replication is Dependent on Nrf 2 16 ARE luciferase activity SFN 50 SFN 40 Count SFN 20 SFN 1 12 8 4 0 SFN NT - 1 5 10 20 15 Nrf 2 (Phospho S 40) V S +V Nrf 2 Nq. O 1 Gclc Gclm Sqstm 1 Hmox-1 MEF Cells A 549 Cells WT Nrf 2 40 -/- Keap 1 -/- 30 20 10 0 SFN - + VSVΔ 51 + + - + + + si. CTRL si. Nrf 2 100 VSV-GFP+ cells (%) S VSV-GFP+ cells (%) NT 50 - + + + 80 60 40 20 0 VSVΔ 51 + +

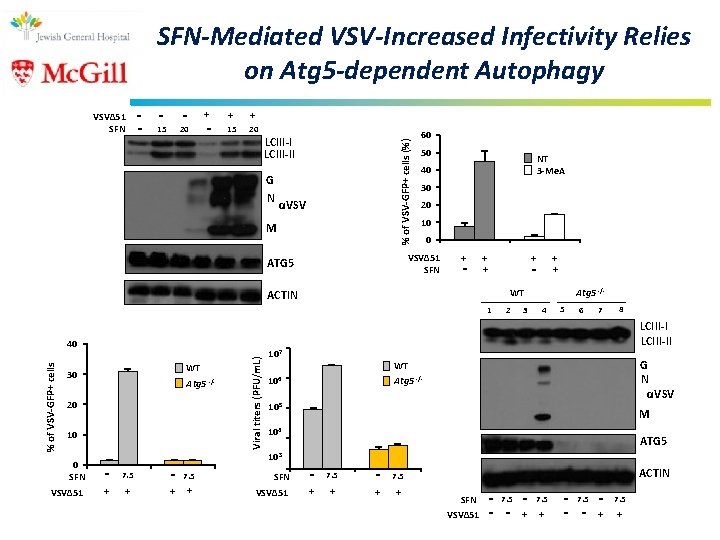
SFN-Mediated VSV-Increased Infectivity Relies on Atg 5 -dependent Autophagy - - - + - 15 20 - + + 15 20 LCIII-II % of VSV-GFP+ cells (%) VSVΔ 51 SFN G N αVSV M 60 50 30 20 10 0 VSVΔ 51 SFN ATG 5 NT 3 -Me. A 40 + - + + + WT ACTIN 1 WT 30 Atg 5 20 10 0 SFN VSVΔ 51 - + -/- Viral titers (PFU/m. L) % of VSV-GFP+ cells 40 + + 2 3 Atg 5 -/4 5 6 7 8 LCIII-II 107 G N αVSV WT Atg 5 106 -/- 105 M 104 ATG 5 103 7. 5 + - 7. 5 SFN + + VSVΔ 51 - + 7. 5 + - + ACTIN 7. 5 + SFN VSVΔ 51 - 7. 5 - - + 7. 5 +

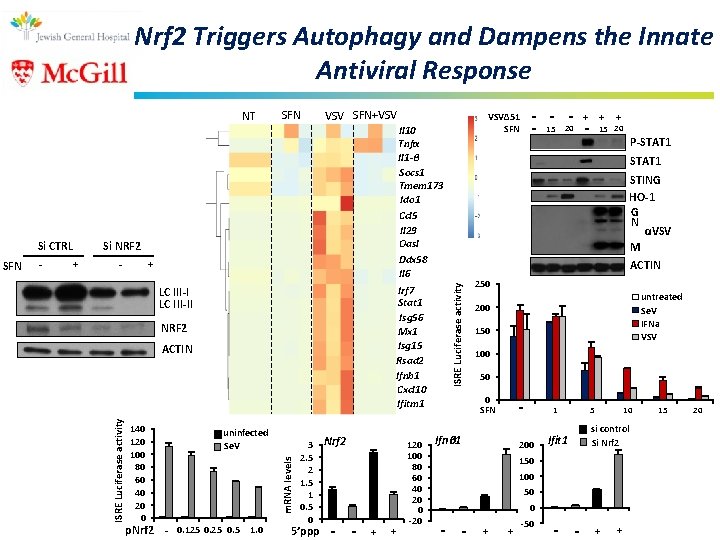
Nrf 2 Triggers Autophagy and Dampens the Innate Antiviral Response - + Il 10 Tnfα Il 1 -β Socs 1 Tmem 173 Ido 1 Ccl 5 Il 29 Oasl Ddx 58 Il 6 Irf 7 Stat 1 Isg 56 Mx 1 Isg 15 Rsad 2 Ifnb 1 Cxcl 10 Ifitm 1 - + LC III-II NRF 2 ACTIN 140 120 100 80 60 40 20 0 uninfected Se. V p. Nrf 2 - 0. 125 0. 5 1. 0 3 Nrf 2 m. RNA levels SFN VSV SFN+VSV Si NRF 2 ISRE Luciferase activity Si CTRL SFN 2. 5 2 1. 5 1 0. 5 0 5’ppp - - + + 120 100 80 60 40 20 0 -20 - - 20 - + - 15 - VSVΔ 51 SFN + + 15 20 P-STAT 1 STING HO-1 G N αVSV M ACTIN ISRE Luciferase activity NT 250 untreated Se. V IFNa VSV 200 150 100 50 0 SFN - Ifnβ 1 1 200 Ifit 1 5 10 si control Si Nrf 2 150 100 50 0 - - + + -50 - - + + 15 20

Conclusions • Stimulation of Nrf 2 using SFN increases VSV-induced oncolysis in various cancer cells • Nrf 2 transcription factor supports VSV replication • SFN potentiation of VSV-induced oncolysis relies on Nrf 2 -dependent autophagy • Activation of Nrf 2 suppresses VSV-induced immune responses (through induction of autophagy ? ? ) • SFN bolstered VSV oncolytic activity in different syngeneic and xenograft mouse models

Acknowledgements Mc. Gill University-Montreal David Olagnier Rasin Lababidi Samar Bel Hadj Yiliu Liu Alexandre Sze Marie-Line Goulet Pasteur Institute - Rome John Hiscott Cindy Chiang Vladimir Beljanski University of Dundee-Dundee Albena Dinkova-Kostova Elena Knatko Fundings
 Potentiate
Potentiate Coherent+
Coherent+ What are capacity defences
What are capacity defences Bipolar analysis of coastal defences
Bipolar analysis of coastal defences Types of criminal law
Types of criminal law Mappleton coastal defences
Mappleton coastal defences Dawlish warren sea defences
Dawlish warren sea defences Defences to negligence
Defences to negligence Antioxidant free radical
Antioxidant free radical Antioxidant classification
Antioxidant classification Vitamin e as antioxidant
Vitamin e as antioxidant Antioxidant system
Antioxidant system Antioxidant means
Antioxidant means Antioxidant classification
Antioxidant classification Antioxidant graphite electrode
Antioxidant graphite electrode Cái miệng bé xinh thế chỉ nói điều hay thôi
Cái miệng bé xinh thế chỉ nói điều hay thôi Chúa sống lại
Chúa sống lại Từ ngữ thể hiện lòng nhân hậu
Từ ngữ thể hiện lòng nhân hậu Tư thế ngồi viết
Tư thế ngồi viết