Medical Device Accident Investigation Seminar TRAINING SEMINAR ON







- Slides: 10

Medical Device Accident Investigation Seminar TRAINING SEMINAR ON MEDICAL DEVICE ACCIDENT INVESTIGATION for Kingdom of Saudi Arabia Saudi Food & Drug Authority Riyadh 11 -14 February, 2007 Mark E. Bruley Vice President, Accident and Forensic Investigation ECRI 5200 Butler Pike, Plymouth Meeting, PA, 19642 USA Tel: [your number] E-mail: [your e-mail address] Web Sites: www. ecri. org www. mdsr. ecri. org ©ECRI 2007 1

©ECRI 2007 2

I. Introduction • • • ECRI Seminar Materials Review Why Investigate Causes of Accidents/Injuries Mechanisms of Injury Accident Investigation Overview Essential Investigation Elements Information Resources Problem Reporting Programmes Investigation Guidelines/Device Testing ©ECRI 2007 3

• • • Nonprofit health services research agency International scope (Offices: USA, UK, UAE, Malaysia) Collaborating Center, World Health Organization Evidence-based Practice Center, Agency for Health Care Policy and Research National Guideline Clearinghouse Interdisciplinary staff of 250 Stringent conflict-of-interest regulations Largest information provider worldwide for healthcare technology — assessment, planning, selection, procurement, management, and risk and quality assessment Consulting support and technical assistance worldwide ©ECRI 2007 4

ECRI Problem Reporting Network • Worldwide voluntary reporting since 1971 • Hospitals, Clinicians, Suppliers • Hazard Investigation and resolution • Educates users and manufacturers • Improves products • Decreases risks ©ECRI 2007 5

ECRI Problem Reporting Network • ECRI Investigates – Analyses Problem – Database Searches – Tests Devices – Contacts Supplier (reviews draft of any reports intended for publication) – Internal and outside review process – Sources remain confidential unless they authorize ECRI to release name. ©ECRI 2007 6

Accident Investigation • Undertaken for hospitals, public health agencies, health professionals, insurers worldwide • Purpose: determine what happened, why, and design prevention techniques • On-site and laboratory investigations • Rapid response by interdisciplinary team ©ECRI 2007 7

©ECRI 2007 8

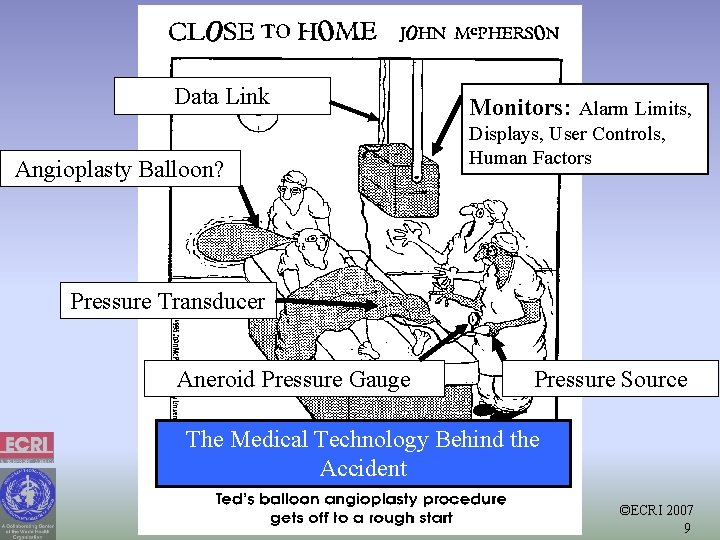
Data Link Angioplasty Balloon? Monitors: Alarm Limits, Displays, User Controls, Human Factors Pressure Transducer Aneroid Pressure Gauge Pressure Source The Medical Technology Behind the Accident ©ECRI 2007 9

The Universe of Medical Devices • • • 5, 000 generic entities 1, 400 capital equipment products 2, 000 surgical instruments 450 implantable devices More than 1, 000 brands, models, and sizes • More than 10, 000 manufacturers ©ECRI 2007 10