MCCN Guidelines on Adult Myeloproliferative Disorders Polycythaemia Vera







- Slides: 8

MCCN Guidelines on Adult Myeloproliferative Disorders Polycythaemia Vera (PV) Diagnostic Criteria 1 JAK 2 -positive PV A 1 High haematocrit (>0. 52 in men, >0. 48 in women) OR raised red cell mass (>25% above predicted)* A 2 Mutation in JAK 2 Diagnosis requires both criteria to be present JAK 2 -negative PV A 1 Raised red cell mass (>25% above predicted) OR haematocrit >0. 60 in men, >0. 56 in women. A 2 Absence of mutation in JAK 2 A 3 No cause of secondary erythrocytosis A 4 Palpable splenomegaly A 5 Presence of an acquired genetic abnormality (excluding BCR-ABL) in the haematopoietic cells B 1 Thrombocytosis (platelet count >450 × 109/l) B 2 Neutrophil leucocytosis (neutrophil count > 10 × 109/l in non-smokers; >12. 5 × 109/l in smokers) B 3 Radiological evidence of splenomegaly B 4 Endogenous erythroid colonies or low serum erythropoietin Diagnosis requires A 1 + A 2 + A 3 + either another A or two B criteria ----------------------------------------*Dual pathology (co-existent secondary erythrocytosis or relative erythrocytosis) may rarely be present in patients with a JAK 2 -positive myeloproliferative disorder. In this situation, it would be prudent to reduce the haematocrit to the same target as for polycythaemia vera. 1

MCCN Guidelines on Adult Myeloproliferative Disorders Polycythaemia Vera (PV) Management 2 There are currently no clinical trials open for patients with PV. It is anticipated that clinical trials will open in due course and appropriate recruitment of eligible patients should be encouraged. In the meantime, the following management recommendations can be made. • conventional risk factors for atherosclerosis (hypertension, hyperlipidaemia, diabetes, smoking) should be managed aggressively. • Venesection to maintain the Hct to <0· 45. • Aspirin 75 mg/d unless contraindicated. • – – – Cytoreduction should be considered if: poor tolerance of venesection symptomatic or progressive splenomegaly other evidence of disease progression, e. g. weight loss, night sweats; thrombocytosis. Choice of cytoreductive therapy, if indicated: <40 years old: first line interferon; second line hydroxycarbamide; third line anagrelide* 40– 75 years old: first line hydroxycarbamide; second line interferon; third line anagrelide* >75 years old: first line hydroxycarbamide; second line 32 P or intermittent low dose busulphan. *anagrelide would require an Individual Funding Application to PCT. Special considerations Thrombosis : These event are relatively common in PV and should be managed by standard guidelines. Risk factors for thrombosis should be meticulously controlled. Bleeding: These complications are relatively uncommon compared with thrombosis. Antiplatelet therapy, or anticoagulants, should be discontinued. The role of epsilon amino caproic acid, tranexamic acid and recombinant VIIa is unclear. Paradoxically, platelet transfusions may be clinically justified. Pregnancy: The local Trust Obstetric lead should be notifed. It is recommended that these patients be notifed to the MPD registry. Management should follow current BCSH guidelines. 2

MCCN Guidelines on Adult Myeloproliferative Disorders Essential Thrombocythaemia (ET) Diagnostic Criteria 3 A 1 Sustained platelet count >450 × 109/l A 2 Presence of an acquired pathogenetic mutation (e. g. in the JAK 2 or MPL genes) A 3 No other myeloid malignancy, especially PV*, PMF†, CML‡ or MDS§ A 4 No reactive cause for thrombocytosis and normal iron stores A 5 Bone marrow aspirate and trephine biopsy showing increased megakaryocyte numbers displaying a spectrum of morphology with predominant large megakaryocytes with hyperlobated nuclei and abundant cytoplasm. Reticulin is generally not increased (grades 0– 2/4 or grade 0/3) Diagnosis requires : A 1–A 3 OR A 1 + A 3–A 5 ----------------------------------------*Excluded by a normal haematocrit in an iron-replete patient; PV, polycythaemia vera. †Indicated by presence of significant marrow bone marrow fibrosis (greater or equal to 2/3 or 3/4 reticulin) AND palpable splenomegaly, blood film abnormalities (circulating progenitors and tear-drop cells) or unexplained anaemia; PMF, primary myelofibrosis. ‡Excluded by absence of BCR-ABL 1 fusion from bone marrow or peripheral blood; CML, chronic myeloid leukaemia. §Excluded by absence of dysplasia on examination of blood film and bone marrow aspirate; MDS, myelodysplastic syndrome. 3

MCCN Guidelines on Adult Myeloproliferative Disorders Essential Thrombocythaemia (ET) Risk Stratification 3 Patients should be stratified according to their risk of thrombotic complications. The most widely accepted risk stratification is as follows: High risk : Patients who are either >60 years of age OR have had an ET-related thrombotic or haemorrhagic event OR who have a platelet count of >1500 × 109/l. Intermediate risk : Patients aged 40– 60 years with no high risk features Low risk: Patients <40 years of age with no high risk features, and Management 3 There are currently no clinical trials open for patients with high risk ET. It is anticipated that clinical trials will open in due course and appropriate recruitment of eligible patients should be encouraged. In the meantime, the following management recommendations can be made. All patient should be offered • • Aggressive management of conventional risk factors for atherosclerosis (hypertension, hyperlipidaemia, diabetes, smoking). Aspirin 75 mg/d unless contraindicated High Risk: Cytoreductive therapy – aim to bring platelet count <400 x 109/l 1 st line therapy : <40 years old - interferon >40 years old - hydroxycarbamide 2 nd line therapy (in those who are refractory or intolerant to 1 st line therapy) ‒ relax platelet count target to 400 -600 x 109/l ‒ Consider alternative cytoreductive agents (including anagrelide, interferon alpha, P 32, busulphan, pipobroman) as either monotherapy or in combination. Non potentially leukaemogenic agents should be used preferentially, if possible. Intermediate Risk/Low risk : Patients should be encouraged to enter the current UK PT 1 trial. Otherwise, they should only be offered cytoreductive therapy if symptomatic with thrombotic (micro- or macrovascular complications) or bleeding complications. 4

MCCN Guidelines on Adult Myeloproliferative Disorders Primary Myelofibrosis (PMF) Diagnostic Criteria 4 JAK 2 positive PMF A 1 Reticulin ≥grade 3 (on a 0 -4 scale) A 2 Mutation of JAK 2 V 617 F B 1 Palpable splenomegaly B 2 Otherwise unexplained anaemia (Hb <11. 5 g/l for men; <10 g/l for women) B 3 Tear drop red cells on PB film B 4 Leucoerythroblastic blood film (presence of at least 2 nucleated red cells or immature myeloid cells in the peripheral blood film) B 5 Systemic symptoms (drenching night sweats, weight loss >10% over 6 months OR diffuse bone pain) B 6 Histological evidence of extramedullary haematopoiesis Diagnosis requires A 1 + A 2 and any two B criteria JAK 2 negative PMF A 1 Reticulin ≥grade 3 (on a 0 -4 scale) A 2 Absence of mutation of JAK 2 V 617 F A 3 Absence of BCR-ABL fusion gene B 1 Palpable splenomegaly B 2 Otherwise unexplained anaemia (Hb <11. 5 g/l for men; <10 g/l for women) B 3 Tear drop red cells on PB film B 4 Leucoerythroblastic blood film (presence of at least 2 nucleated red cells or immature myeloid cells in the peripheral blood film) B 5 Systemic symptoms (drenching night sweats, weight loss >10% over 6 months OR diffuse bone pain) B 6 Histological evidence of extramedullary haematopoiesis Diagnosis requires A 1 + A 2 and any two B criteria 5

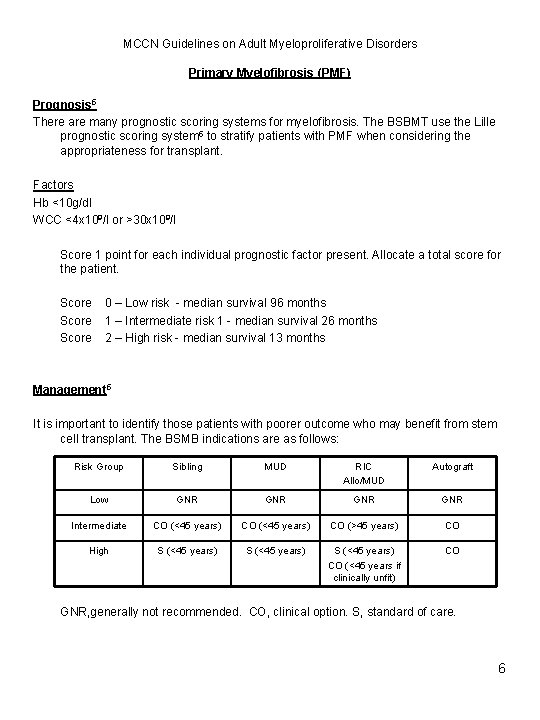
MCCN Guidelines on Adult Myeloproliferative Disorders Primary Myelofibrosis (PMF) Prognosis 5 There are many prognostic scoring systems for myelofibrosis. The BSBMT use the Lille prognostic scoring system 5 to stratify patients with PMF when considering the appropriateness for transplant. Factors Hb <10 g/dl WCC <4 x 109/l or >30 x 109/l Score 1 point for each individual prognostic factor present. Allocate a total score for the patient. Score 0 – Low risk - median survival 96 months 1 – Intermediate risk 1 - median survival 26 months 2 – High risk - median survival 13 months Management 5 It is important to identify those patients with poorer outcome who may benefit from stem cell transplant. The BSMB indications are as follows: Risk Group Sibling MUD RIC Allo/MUD Autograft Low GNR GNR Intermediate CO (<45 years) CO (>45 years) CO High S (<45 years) CO (<45 years if clinically unfit) CO GNR, generally not recommended. CO, clinical option. S, standard of care. 6

MCCN Guidelines on Adult Myeloproliferative Disorders Primary Myelofibrosis (PMF) Management In those patients not suitable for transplant, it is hoped that clinical trials in PMF will open nationally. Until such triathe aim is to treat constitutional symptoms and reduce transfusion requirement. The following options should be considered. 1. Supportive therapy – blood product support. 2. Cytoreductive agents – especially in those with constitutional symptoms / progressive splenomegaly / leucocytosis or thrombocytosis / bone pain: 1 st line – Hydroxycarbamide; alternatives include busulphan, melphalan, purine analogues. 3. Erythropoietin: as per local protocol. 4. Androgens: e. g danazol. Dose is weight dependent – <80 kg 300 mg bd, >80 kg 400 mg bd. Minimum treatment period 6 months. Those responding (Hb >1. 5 g/dl with transfusion independence) should continue danazol at dose 200 mg bd for further 6 months, followed by stepwise dose reductions until minimum dose maintaining response is achieved. Stop danazol if failing to respond. 5. Thalidomide - low dose (50 mg daily) and prednisolone (0. 5 mg-1 mg/kg – tapered over 12 weeks). Median time to response 7. 5 weeks (2 - 15 weeks). Response rate 77%. 6. Splenomegaly : Splenectomy - indications - symptomatic splenomegaly refractory to treatment - severe constitutional symptoms - uncontrollable haemolysis - transfusion dependent anaemia unresponsive to treatment - portal hypertension - marked thrombocytopenia Splenic irradiation for those deemed unfit for surgery. 7

MCCN Guidelines on Adult Myeloproliferative Disorders References 1. Amendment to the diagnosis and investigation of polycythaemia/erythrocytosis. British Journal of Haematology 2007; 138 (6): 821 -2 2. Guidelines for the Diagnosis, Investigation and Management of Polycythaemia/Erythrocytosis. British Journal of Haematology 2005; 130(2): 174 -95 3. Guideline for investigation and management of adults and children presenting with a thrombocytosis. British Journal of Haematology 2010; 149 (3) 352 -375 4. Proposed criteria for PMF in upcoming COSMYD trials (Collaborative Study in Myeloproliferative Disorders). 5. Prognostic factors in agnogenic myeloid metaplasia: a report on 195 cases with a new scoring system. Blood. 1996 Aug 1; 88(3): 1013 -8. Prepared by Dr N M Butt On behalf of the MCCN Haematology Cancer Network Group. 16 th July 2010. 8