Liquids Ethanol also called alcohol ethyl alcohol and



![Properties[edit] Isopropyl alcohol is miscible in water, ethanol, ether, and chloroform. It will dissolve Properties[edit] Isopropyl alcohol is miscible in water, ethanol, ether, and chloroform. It will dissolve](https://slidetodoc.com/presentation_image/ca52473c8594a8572fd44b646fa75c7b/image-5.jpg)
- Slides: 6

Liquids

• Ethanol, also called alcohol, ethyl alcohol, and drinking alcohol, is the principal type of alcohol found in alcoholic beverages. It is a volatile, flammable, colorless liquid with a slight characteristic odor. • Ethanol is mostly produced by the fermentation of sugars by yeasts, or by petrochemical processes. • It is an addictive psychoactive drug (indeed one of the oldest and most common recreational drugs), causing a characteristic intoxication ("drunkenness") and neurotoxicity when consumed in sufficient quantities. [15][16] It is • widely used as a solvent, as fuel, and as a feedstock for synthesis of other chemicals; as well as in many other minor uses. • The largest single use of ethanol is as an engine fuel and fuel additive. • Methyl Alcohol a colorless, volatile, water-soluble, poisonous liquid, CH 4 O, obtained by the destructive distillation of wood or the incomplete oxidation of natural gas, or produced synthetically from carbon monoxide and hydrogen, • used chiefly as a solvent, a fuel, and an automobile antifreeze and in the synthesis of formaldehyde • Isopropyl alcohol is a colorless, combustible liquid with a wide variety of uses. It has a wide range of uses in the home and is used in laboratories, in medicine, and in many manufacturing industries. Two of its most popular uses are as a solvent and a cleaning fluid. • is a major ingredient in "gas dryer" fuel additives • Propene is one of the basic materials necessary to produce isopropyl alcohol. This compound comes from fossil fuels--petroleum, natural gas and even coal • n-Butyl alcohol is a highly refractive, flammable, colorless liquid, which burns with a luminous flame. It has an odor similar to bananas and fuel oil. n-Butyl alcohol is incompatible with aluminum, chromium trioxide, and oxidizing materials. It is miscible with alcohol, ether, and many other organic solvents (Merck, 1989; Sax, 1989). n-Butanol has been proposed as a substitute for diesel fuel and gasoline

Ethanol, also called alcohol, ethyl alcohol, and drinking alcohol, is the principal type of alcohol found in alcoholic beverages. It is a volatile, flammable, colorless liquid with a slight characteristic odor. Its chemical formula is C 2 H 6 O, which can be written also as CH 3 -CH 2 -OH or C 2 H 5 -OH (an ethyl group linked to a hydroxyl group), and is often abbreviated as Et. OH. Ethanol is mostly produced by the fermentation of sugars by yeasts, or by petrochemical processes. It is an addictive psychoactive drug (indeed one of the oldest and most common recreational drugs), causing a characteristic intoxication ("drunkenness") and neurotoxicity when consumed in sufficient quantities. [15][16] It is widely used as a solvent, as fuel, and as a feedstock for synthesis of other chemicals; as well as in many other minor uses. Ethanol was commonly used as fuel in early bipropellant rocket (liquid propelled) vehicles, in conjunction with an oxidizer such as liquid oxygen. Ethanol is miscible with water and is a good general purpose solvent. The largest single use of ethanol is as an engine fuel and fuel additive.

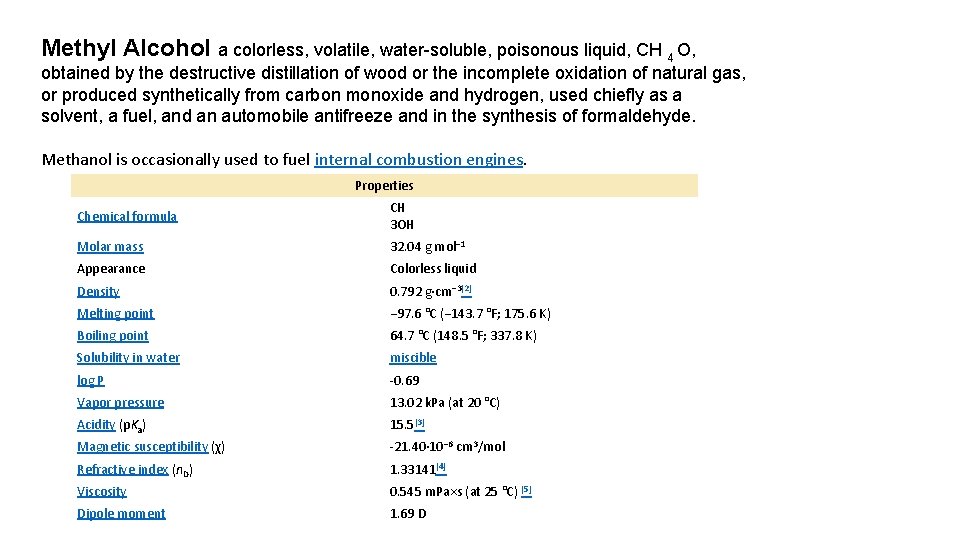
Methyl Alcohol a colorless, volatile, water-soluble, poisonous liquid, CH 4 O, obtained by the destructive distillation of wood or the incomplete oxidation of natural gas, or produced synthetically from carbon monoxide and hydrogen, used chiefly as a solvent, a fuel, and an automobile antifreeze and in the synthesis of formaldehyde. Methanol is occasionally used to fuel internal combustion engines. Properties Chemical formula CH 3 OH Molar mass 32. 04 g mol− 1 Appearance Colorless liquid Density 0. 792 g·cm− 3[2] Melting point − 97. 6 °C (− 143. 7 °F; 175. 6 K) Boiling point 64. 7 °C (148. 5 °F; 337. 8 K) Solubility in water miscible log P -0. 69 Vapor pressure 13. 02 k. Pa (at 20 °C) Acidity (p. Ka) 15. 5[3] Magnetic susceptibility (χ) -21. 40· 10− 6 cm 3/mol Refractive index (n. D) 1. 33141[4] Viscosity 0. 545 m. Pa×s (at 25 °C) [5] Dipole moment 1. 69 D
![Propertiesedit Isopropyl alcohol is miscible in water ethanol ether and chloroform It will dissolve Properties[edit] Isopropyl alcohol is miscible in water, ethanol, ether, and chloroform. It will dissolve](https://slidetodoc.com/presentation_image/ca52473c8594a8572fd44b646fa75c7b/image-5.jpg)
Properties[edit] Isopropyl alcohol is miscible in water, ethanol, ether, and chloroform. It will dissolve ethyl cellulose, polyvinyl butyral, many oils, alkaloids, gums and natural resins. [6] Unlike ethanol or methanol, isopropyl alcohol is not miscible with salt solutions and can be separated from aqueous solutions by adding a salt such as sodium chloride. The process is colloquially called salting out, and causes concentrated isopropyl alcohol to separate into a distinct layer. [7] Isopropyl alcohol forms an azeotrope with water, which gives a boiling point of 80. 37 °C (176. 67 °F) and a composition of 87. 7 wt% (91 vol%) isopropyl alcohol. Water-isopropyl alcohol mixtures have depressed melting points. [7] It has a slightly bitter taste, and is not safe to drink. [7][8] Isopropyl alcohol becomes increasingly viscous with decreasing temperature will freeze at − 89 °C (− 128 °F). Isopropyl alcohol has a maximum absorbance at 205 nm in an ultraviolet-visible spectrum. [9][10] Isopropyl alcohol is a major ingredient in "gas dryer" fuel additives Properties Chemical formula C 3 H 8 O Molar mass 60. 10 g·mol− 1 Appearance Colorless liquid Density 0. 786 g/cm 3 (20 °C) Melting point − 89 °C (− 128 °F; 184 K) Boiling point 82. 6 °C (180. 7 °F; 355. 8 K) Solubility in water miscible in water Solubility miscible in benzene, chloroform, ethanol, ether, glycerin soluble in acetone Acidity (p. Ka) 16. 5[2] Magnetic susceptibility (χ) -45. 794· 10− 6 cm 3/mol Refractive index (n. D) 1. 3776 Viscosity 2. 86 c. P at 15 °C 1. 96 c. P at 25 °C[3] 1. 77 c. P at 30 °C[3] Dipole moment 1. 66 D (gas

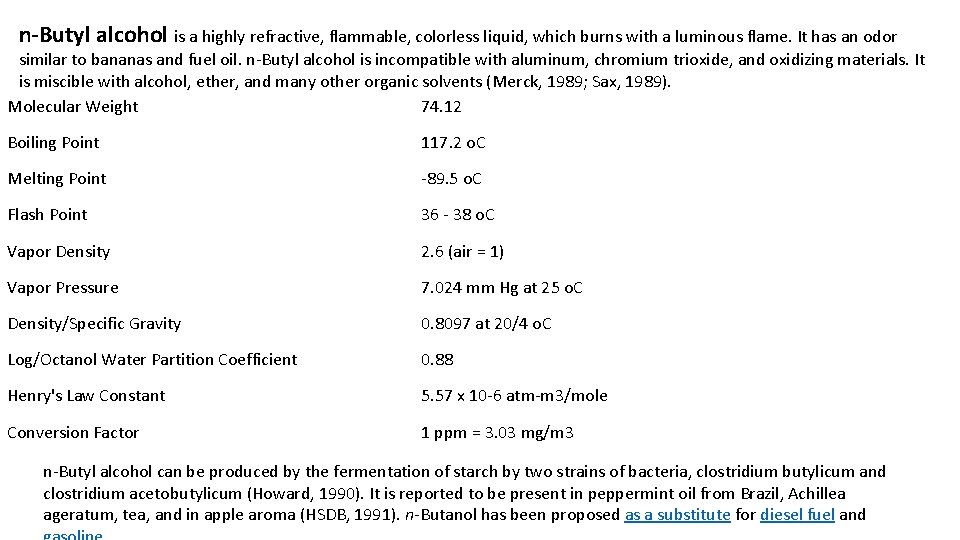
n-Butyl alcohol is a highly refractive, flammable, colorless liquid, which burns with a luminous flame. It has an odor similar to bananas and fuel oil. n-Butyl alcohol is incompatible with aluminum, chromium trioxide, and oxidizing materials. It is miscible with alcohol, ether, and many other organic solvents (Merck, 1989; Sax, 1989). Molecular Weight 74. 12 Boiling Point 117. 2 o. C Melting Point -89. 5 o. C Flash Point 36 - 38 o. C Vapor Density 2. 6 (air = 1) Vapor Pressure 7. 024 mm Hg at 25 o. C Density/Specific Gravity 0. 8097 at 20/4 o. C Log/Octanol Water Partition Coefficient 0. 88 Henry's Law Constant 5. 57 x 10 -6 atm-m 3/mole Conversion Factor 1 ppm = 3. 03 mg/m 3 n-Butyl alcohol can be produced by the fermentation of starch by two strains of bacteria, clostridium butylicum and clostridium acetobutylicum (Howard, 1990). It is reported to be present in peppermint oil from Brazil, Achillea ageratum, tea, and in apple aroma (HSDB, 1991). n-Butanol has been proposed as a substitute for diesel fuel and










