Light Where does light come from Light is





- Slides: 16

Light!

Where does light come from? • Light is the result of _________________ from an energized (usually heated) body of matter. Most often thought of as “visible light”, but electromagnetic radiation occurs over a large _______ of wavelengths.

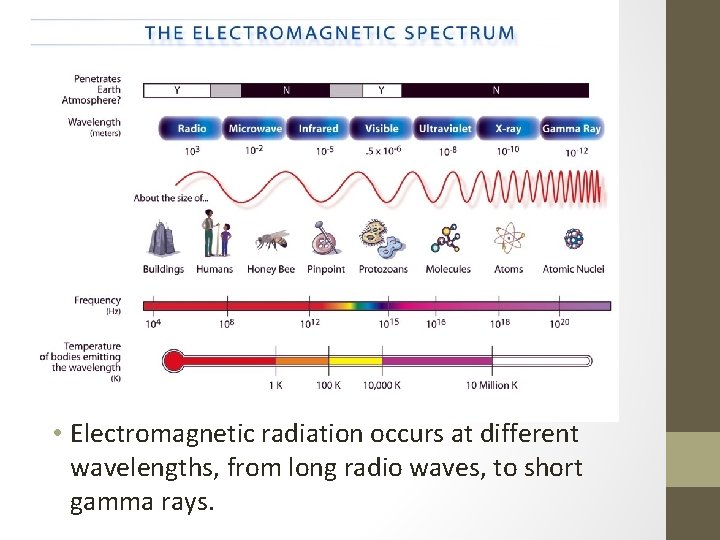
• Electromagnetic radiation occurs at different wavelengths, from long radio waves, to short gamma rays.

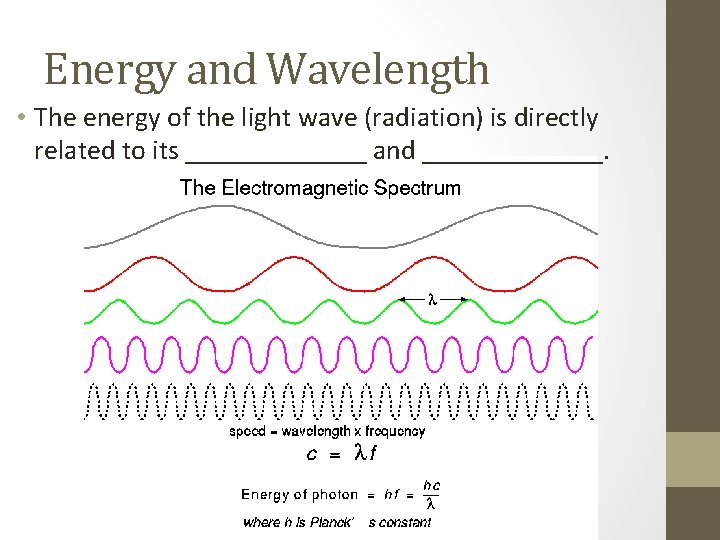
Energy and Wavelength • The energy of the light wave (radiation) is directly related to its _______ and _______.

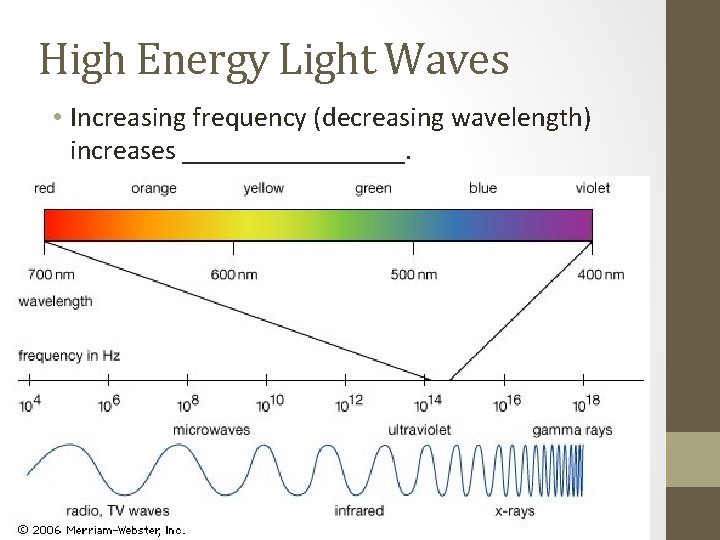
High Energy Light Waves • Increasing frequency (decreasing wavelength) increases ________.

Chemistry and Light • We have already seen that light emission results from electrons during the excitation from and emission of radiation.

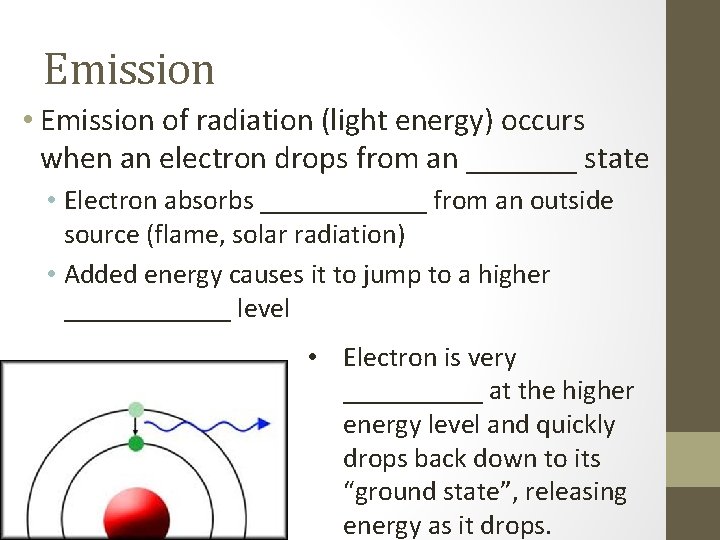
Emission • Emission of radiation (light energy) occurs when an electron drops from an _______ state • Electron absorbs ______ from an outside source (flame, solar radiation) • Added energy causes it to jump to a higher ______ level • Electron is very _____ at the higher energy level and quickly drops back down to its “ground state”, releasing energy as it drops.

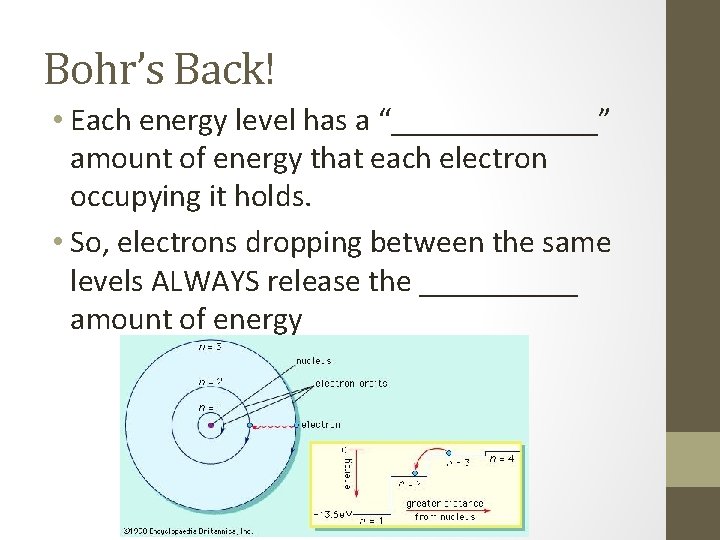
Bohr’s Back! • Each energy level has a “_______” amount of energy that each electron occupying it holds. • So, electrons dropping between the same levels ALWAYS release the _____ amount of energy

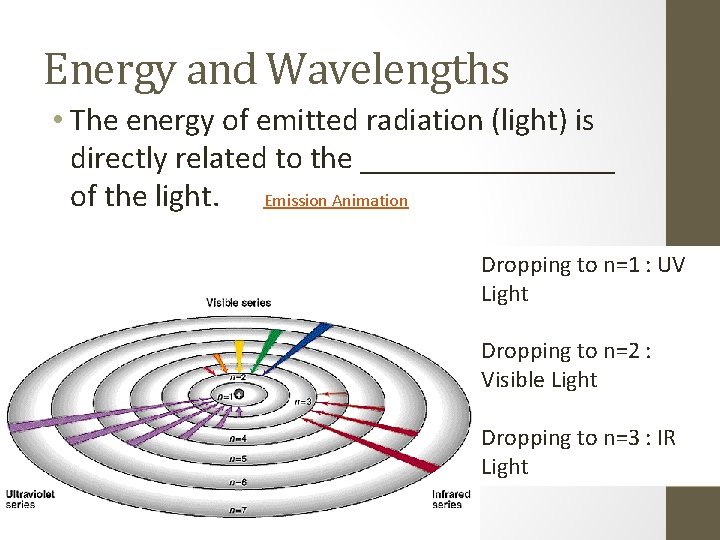
Energy and Wavelengths • The energy of emitted radiation (light) is directly related to the ________ of the light. Emission Animation Dropping to n=1 : UV Light Dropping to n=2 : Visible Light Dropping to n=3 : IR Light

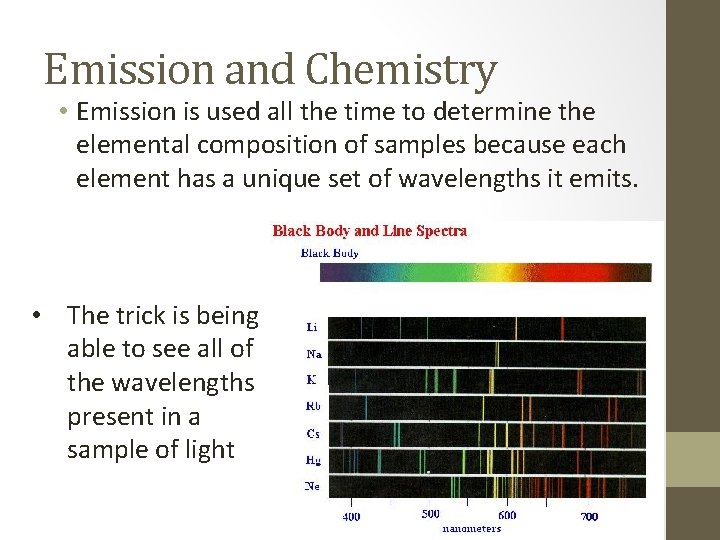
Emission and Chemistry • Emission is used all the time to determine the elemental composition of samples because each element has a unique set of wavelengths it emits. • The trick is being able to see all of the wavelengths present in a sample of light

Flame Test • Gave signature “colors” of emission for different elements • Limits • Only useful for metals • Need a lot of sample to heat to see the color • Only see color that results from mixture of all the wavelengths present. (What if 2 are orange? !? )

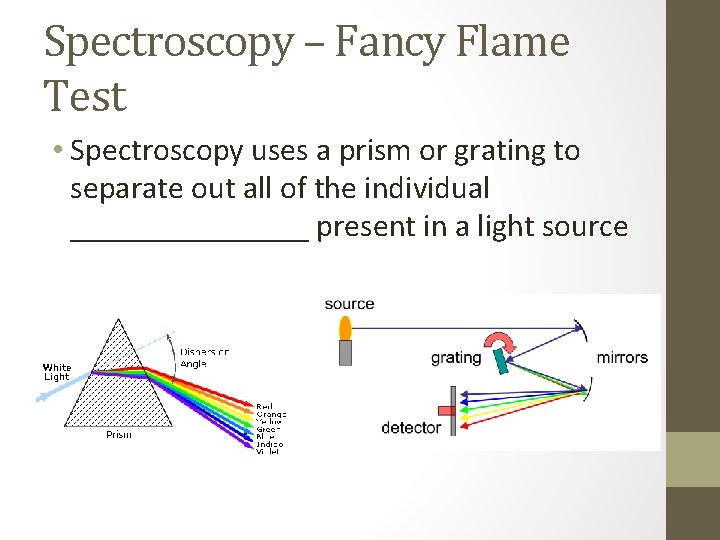
Spectroscopy – Fancy Flame Test • Spectroscopy uses a prism or grating to separate out all of the individual ________ present in a light source

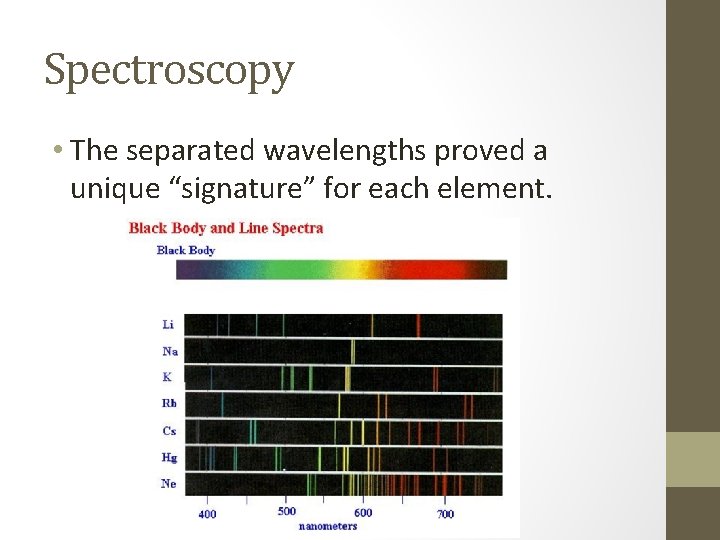
Spectroscopy • The separated wavelengths proved a unique “signature” for each element.

Applications of Spectroscopy • Determining the elements present in unknown samples • Mars Rover (soil/rock samples)

Applications of Spectroscopy • Determining the elements present in celestial bodies • Stars, Gas Clouds

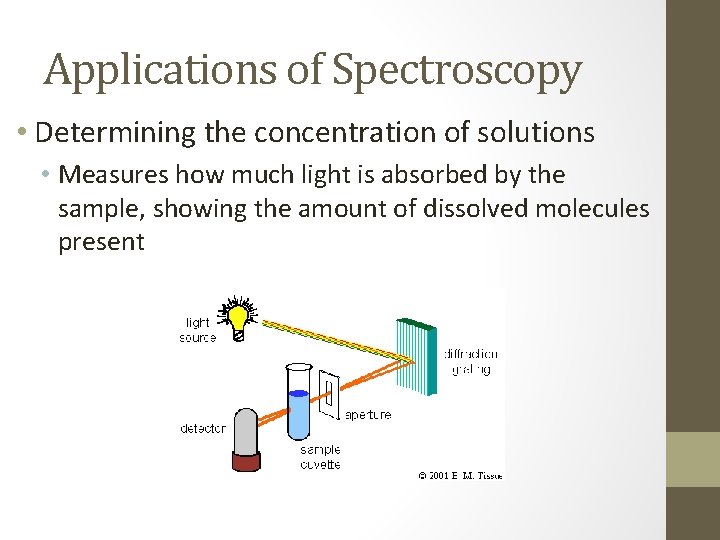
Applications of Spectroscopy • Determining the concentration of solutions • Measures how much light is absorbed by the sample, showing the amount of dissolved molecules present