Levonorgestrel intrauterine contraceptive systems 13 5 mg and






- Slides: 9

Levonorgestrel intrauterine contraceptive systems (13. 5 mg and 52 mg) and risk of ectopic pregnancy Sofie Graner 1, 2 |Julia Mc Taggart 3 | Fanny Nordström 3 | | Emma Melander 3 Johan Widenberg 4| Helena Kopp Kallner 3, 4, 5 1: Department of Medicine, Center for Pharmacoepidemiology, Karolinska Institutet , Karolinska University Hospital, Stockholm, Sweden; 2: Maternity BB Stockholm, Danderyds Hospital, Stockholm, Sweden; 3: Department of Clinical Sciences, Danderyd Hospital, Karolinska Institute, Stockholm, Sweden, 4: Department of Obstetrics and Gynecology, Danderyd Hospital, Stockholm, Sweden; 5: Department of Women's and Children's Health, Karolinska Institute, Stockholm, Sweden ACTA Obstetricia et Gynecologica Scandinavica Journal Club July 2019 Edited by Francesco D’Antonio

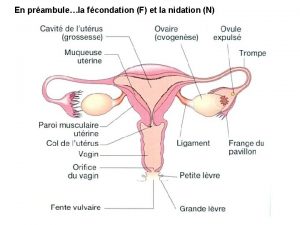
Introduction Ø Approximately 2% of all pregnancies are ectopic pregnancies (EP), and EP is the leading cause of maternal mortality in the first trimester, accounting for approximately 9% of the maternal mortality in the USA. Ø Risk factors for EP include previous history of EP, surgery, infection of the pelvic organs (including Chlamydia trachomatis), in vitro fertilization, smoking, and also use of intrauterine contraception. These are generally not considered contraindications for use of intrauterine contraception due to the high contraceptive efficacy of the current devices. Ø In recent years a smaller levonorgestrel-containing intrauterine system (LNG-IUS 13. 5 mg, total LNG reservoir content 13. 5 mg) has been marketed. Ø Recently published studies reported that the LNG-IUS 13. 5 mg provided well-tolerated and effective contraception. However, among the reported pregnancies, 50% were EP.

Aim of the study To investigate the Pearl index for ectopic pregnancy in women using the levonorgestrel intrauterine system (LNG-IUS) at the time of conception.

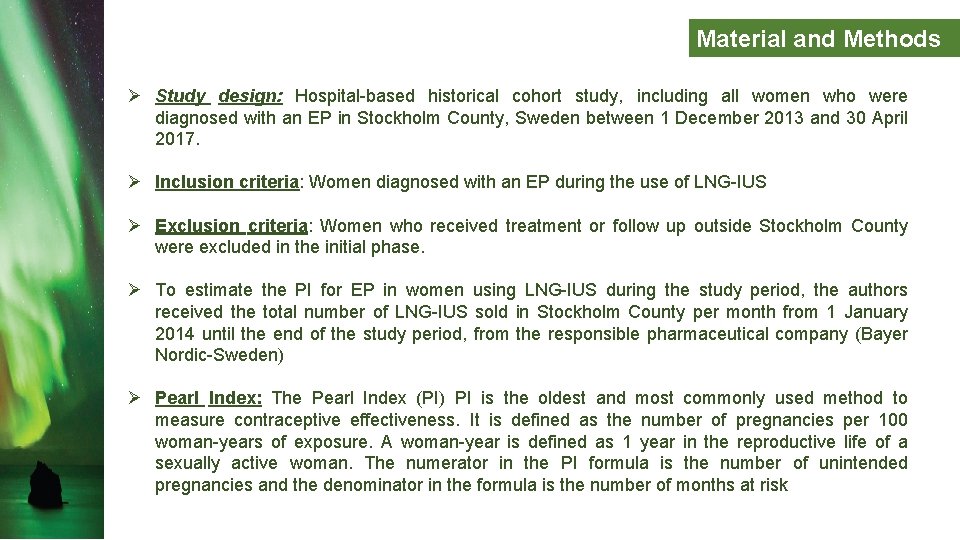
Material and Methods Ø Study design: Hospital-based historical cohort study, including all women who were diagnosed with an EP in Stockholm County, Sweden between 1 December 2013 and 30 April 2017. Ø Inclusion criteria: Women diagnosed with an EP during the use of LNG-IUS Ø Exclusion criteria: Women who received treatment or follow up outside Stockholm County were excluded in the initial phase. Ø To estimate the PI for EP in women using LNG-IUS during the study period, the authors received the total number of LNG-IUS sold in Stockholm County per month from 1 January 2014 until the end of the study period, from the responsible pharmaceutical company (Bayer Nordic-Sweden) Ø Pearl Index: The Pearl Index (PI) PI is the oldest and most commonly used method to measure contraceptive effectiveness. It is defined as the number of pregnancies per 100 woman-years of exposure. A woman-year is defined as 1 year in the reproductive life of a sexually active woman. The numerator in the PI formula is the number of unintended pregnancies and the denominator in the formula is the number of months at risk

Material and Methods Ø Statistical analysis: The PI was calculated by the number of EP per 100 participant years with 95% CI calculations based on the assumption that the number of EPs followed a Poisson distribution. .

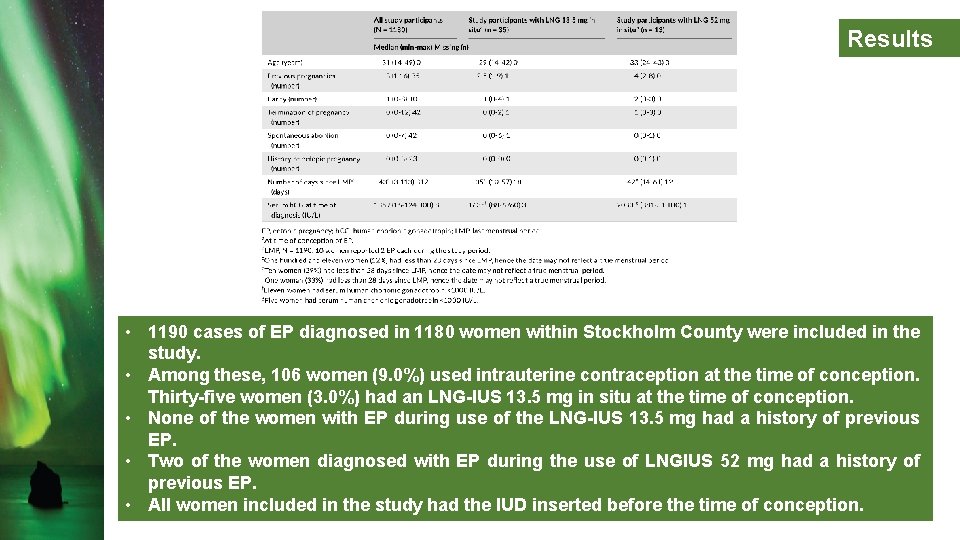
Results • 1190 cases of EP diagnosed in 1180 women within Stockholm County were included in the study. • Among these, 106 women (9. 0%) used intrauterine contraception at the time of conception. Thirty-five women (3. 0%) had an LNG-IUS 13. 5 mg in situ at the time of conception. • None of the women with EP during use of the LNG-IUS 13. 5 mg had a history of previous EP. • Two of the women diagnosed with EP during the use of LNGIUS 52 mg had a history of previous EP. • All women included in the study had the IUD inserted before the time of conception.

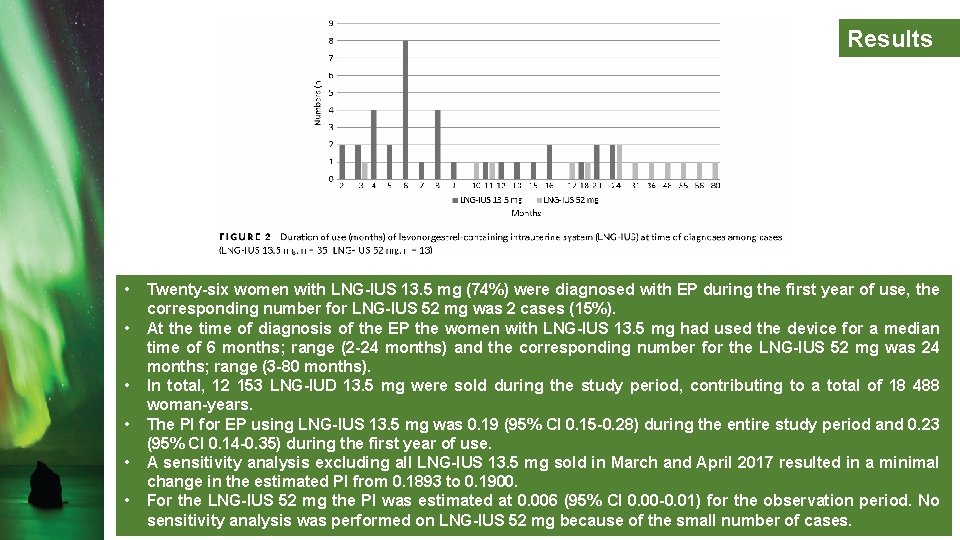
Results • • • Twenty-six women with LNG-IUS 13. 5 mg (74%) were diagnosed with EP during the first year of use, the corresponding number for LNG-IUS 52 mg was 2 cases (15%). At the time of diagnosis of the EP the women with LNG-IUS 13. 5 mg had used the device for a median time of 6 months; range (2 -24 months) and the corresponding number for the LNG-IUS 52 mg was 24 months; range (3 -80 months). In total, 12 153 LNG-IUD 13. 5 mg were sold during the study period, contributing to a total of 18 488 woman-years. The PI for EP using LNG-IUS 13. 5 mg was 0. 19 (95% CI 0. 15 -0. 28) during the entire study period and 0. 23 (95% CI 0. 14 -0. 35) during the first year of use. A sensitivity analysis excluding all LNG-IUS 13. 5 mg sold in March and April 2017 resulted in a minimal change in the estimated PI from 0. 1893 to 0. 1900. For the LNG-IUS 52 mg the PI was estimated at 0. 006 (95% CI 0. 00 -0. 01) for the observation period. No sensitivity analysis was performed on LNG-IUS 52 mg because of the small number of cases.

Limitations Ø Retrospective non-randomized design Ø Heterogeneity in the diagnosis of EP Ø Lack of consistent follow-up

Conclusion The absolute risk of EP during the use of LNG-IUS 13. 5 mg is low