ISO TC 215 IEEE 11073 Joint Topics Agenda










- Slides: 12

ISO TC 215 / IEEE 11073 Joint Topics

Agenda 1. 2. 3. 4. 5. 6. Introductions Check on current PSDO agreement: general content Titles of IEEE 11073 series Revision of adopted IEEE 11073 standards Resolve Comments ISO/IEEE 11073 -10102 Resolve Comments to IEEE 11073 -10103 SR

2. Review of Current PSDO Agreement • Up for renewal • Every 3 years • Current agreement stays in place until new version signed off

3. Titles of IEEE 11073 Series • ISO requests that all 11073 Standards have the same prefix, with modifiers such as Personal Health Device or Point-of-Care Device incorporated standard specific part. • Currently the IEEE 11073 Series Titles follow this format: • ISO/IEEE 11073 -10101: 2004 -- Health informatics – Point-of-care medical device communication – Part 10101: Nomenclature • Proposal to ISO would be to follow this format: • ISO/IEEE 11073 -10101: 2004 -- Health informatics – Device interoperability -Part 10101: Point-of-care medical device communication – Nomenclature

3. Titles of IEEE 11073 Series • Part titles can be mapped directly from the IEEE version in a way that maintains clarity (e. g. , Po. CD vs. PHD) and continuity - if you are used to looking at the IEEE project title then see the ISO/CEN title. . . there shouldn't be any dissonance • Additional discussion within IEEE would be whether to change the IEEE titles as they get revised. • Strong incentive to align titles – should we modify existing PARs? • In the meantime, how do we go about changing the ISO titles? • ISO titles are already being changed as the documents are revised or published.

4. Revision of adopted IEEE 11073 standards • What do we do to respond to comments from ISO? • Definitely need to formally respond to ISO comments. • Up to IEEE concerning whether to revise the document and the timing of document revision.

5 a. Editorial Comments ISO/IEEE 11073 -10102 • SR copy had many “typos” – potentially conversion issues. • Unclear whether the issue was just in the distribution copy or in the actual downloadable ISO version of the standard. • ISO may revise their version if the issue is indeed in the published copy. • Timeframe for an IEEE update is 2” years. • Proposed Response: • Unfortunately during the ISO publishing process errors were introduced which caused the easily identified but incorrect text. We have checked other documents and have not uncovered other cases. The text will be corrected once 10102 is updated by IEEE and approved by ISO/TC 215. • Comment 1, 2, 3, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15

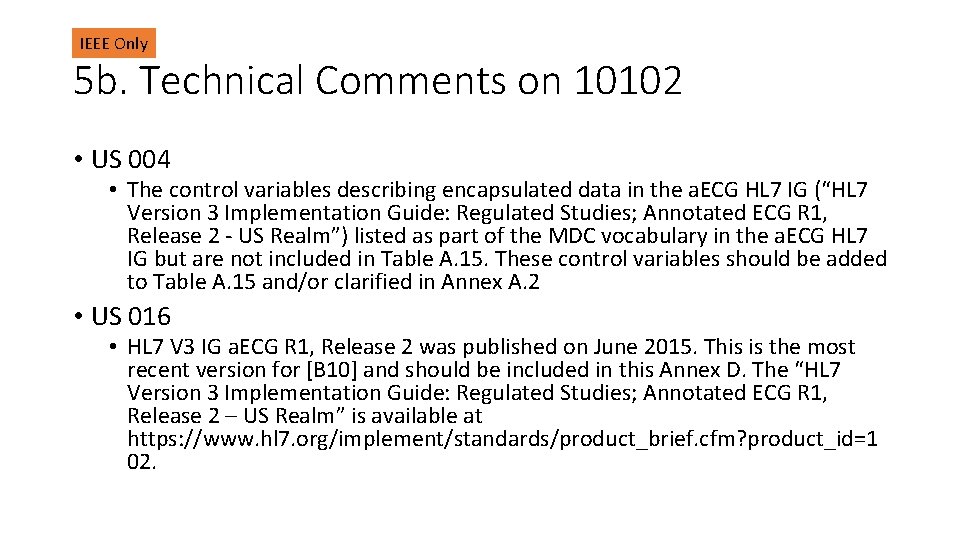
IEEE Only 5 b. Technical Comments on 10102 • US 004 • The control variables describing encapsulated data in the a. ECG HL 7 IG (“HL 7 Version 3 Implementation Guide: Regulated Studies; Annotated ECG R 1, Release 2 - US Realm”) listed as part of the MDC vocabulary in the a. ECG HL 7 IG but are not included in Table A. 15. These control variables should be added to Table A. 15 and/or clarified in Annex A. 2 • US 016 • HL 7 V 3 IG a. ECG R 1, Release 2 was published on June 2015. This is the most recent version for [B 10] and should be included in this Annex D. The “HL 7 Version 3 Implementation Guide: Regulated Studies; Annotated ECG R 1, Release 2 – US Realm” is available at https: //www. hl 7. org/implement/standards/product_brief. cfm? product_id=1 02.

IEEE Only 5 b. Technical Comments on 10102 • US 017 • Release 2 of HL 7 V 3 IG a. ECG was updated to use _AVR, _AVL, and _AVF. The last sentence of the first paragraph should be deleted. • US 018 • NOTE 2 should be updated to reference HL 7 V 3 IG a. ECG R 1, Release 2 (see comment above). The last sentence should be deleted. • US 019 • The most recent version for [B 10] is “HL 7 Version 3 Implementation Guide: Regulated Studies; Annotated ECG R 1, Release 2 - US Realm” is available at https: //www. hl 7. org/implement/standards/product_brief. cfm? product_id=1 02.

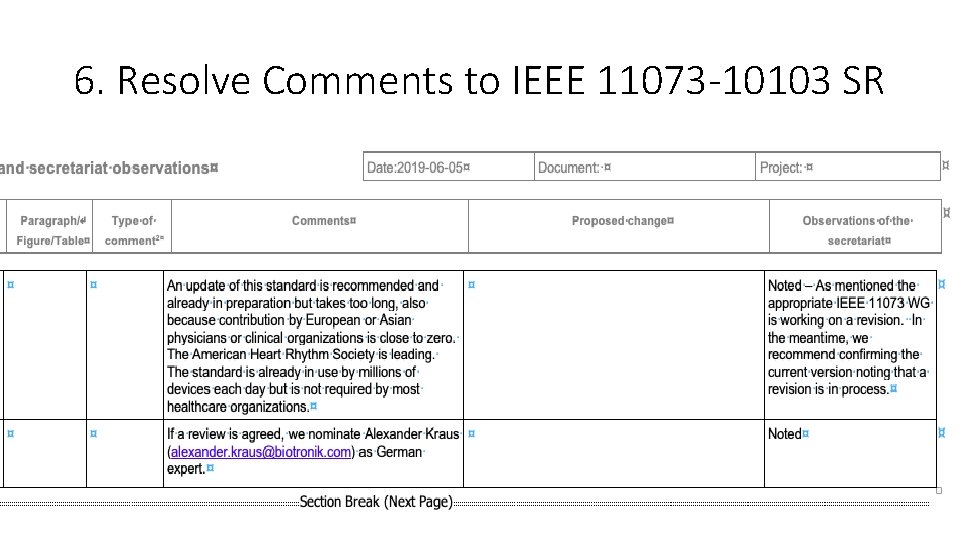
6. Comments to ISO/IEEE 11073 -10103 SR • Response from Alexander Kraus – who originated the DE comment: • It was a misunderstanding with the German DIN. Go ahead to 'confirm the current version of the Standard'. It's the best we have today which will also be required by the way starting Jan 2020 for reimbursement in Germany! The response was just trying to support the actual PAR (we probably have to extend again) and activity at IDCO to update the standard which needs an update regarding episode types for example but also more strict data representation (!) rules. I can not give you a reliable new date but don't expect an update to V 1. 1 before 2021.

6. Resolve Comments to IEEE 11073 -10103 SR • Proposed Response • As noted in the comment, work is progressing within the appropriate IEEE 11073 Sub-Group. Interested experts are very welcome to contribute.

7. Resolve Comments to IEEE 11073 -10201 SR