Implementation of Disclosure Legislation in Massachusetts National Disclosure























- Slides: 26

Implementation of Disclosure Legislation in Massachusetts National Disclosure Summit March 6, 2009 Allan Coukell, Director of Policy

Sources of funding: The Pew Charitable Trusts Conflicts of interest: none

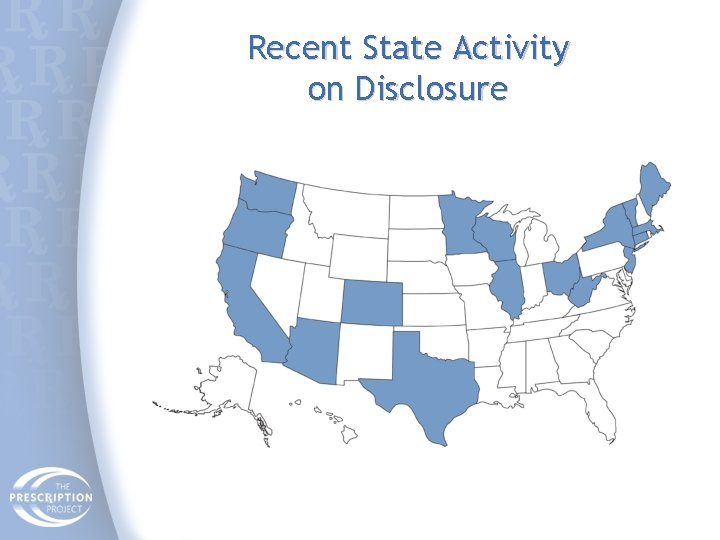
Recent State Activity on Disclosure

Current State Laws on Disclosure • Minnesota (1993): Public disclosure of all payments over $100. Ban on gifts over $50; education and other exemptions. • Vermont (2002): Disclosure of individual MD payments to Attorney General; aggregated for public. • Maine (2003): Not yet implemented. Non-public disclosure of individual payments >$25. • West Virginia (2004): Broad disclosure but no individual MD names. • Washington DC (2005): Non-public disclosure. Annual reports to DPH. • Massachusetts (2008, rules pending): Public disclosure of all payments except research. Ban on gifts, meals, entertainment, travel & expenses.

Physician Payments Sunshine Act

Past Action in MA • 2005 -6 Fair Pricing Act –Sponsored by Sen. Montigny –Discount program for seniors –Statewide uniform PDL, PA protections, bulk purchasing –Gifts ban and disclosure –Did not pass

Massachusetts Cost & Quality Law

Massachusetts Cost & Quality Law • Requires DPH to write a marketing code of conduct that is "no less restrictive" than Ph. RMA and Adva. Med codes • Requires public disclosure of all payments over $50 made to doctors (with certain exclusions) • Funds an evidence-based prescriber education program

Cost & Quality Timeline • Jan 07: MA Cost and Quality bill initially introduced • Feb 08: Reintroduction. MA Senate President files 17 -point cost and quality bill including disclosure and gifts ban and academic detailing program • Apr 08: C&Q bill passed Senate with gifts ban and prescriber education • Prescriber education in final budget • Jul 08: C&Q bill passed House with prescriber education; no gifts ban or disclosure; industry codes to be submitted; ban on data mining • In Conference, gifts ban, disclosure, and prescriber education are preserved, data mining ban is dropped. • Aug 08: Bill passes legislature and is signed into law • Dec 08: DPH releases set of draft regulations.

C&Q Law: Gifts & Meals • Sponsored meals must accompany an informational presentation and must be conducted in the doctors office or hospital setting. • Industry may not pay for meals that are part of a recreational event. • Industry may not provide any recreational items.

C&Q Law : CME & Events • Industry may not sponsor CME that does not meet ACCME standards. • Industry may not provide payment for attendance or travel support to attendees of off-site CME or other events. • Industry may not provide direct payment for meals during CME, professional meetings, and events.

C&Q Law: Allowed • Distribution of peer-reviewed information • Samples for patient use • Compensation for substantial professional or consulting services in conjunction with research or a clinical trial • Training on devices

C&Q Law: Disclosure • Annual disclosure of payments to prescribers/ purchasers. Made public on a searchable website. • What: fee, payment, subsidy, other economic benefit Greater than $50, provided directly or through a company’s agents • To: Physician, hospital, nursing home, pharmacist, health benefit plan administrator, heath care practitioner, any other authorized to prescribe, dispense or purchase prescription drugs or devices. • Including: value, nature, purpose, particular recipient • Funding: user-fee based (to be established by DPH) • Enforcement: DPH will report any payment in violation of the code of conduct to the AG. Penalties of not more than $5000 per violating transaction

C&Q Law: Industry must… • Institute mechanisms for complying with the code of conduct by July 1, 2009 • Provide a regular training program for employees on code of conduct compliance • Conduct annual audits to monitor compliance among employees • Adopt policies and procedures for investigating noncompliance and take corrective action • Identify a compliance officer for operating and monitoring code of conduct • Submit an annual report to DPH including: a description of its training program; name and contact for its compliance officer; and certification it has completed its annual audit.

DPH Draft Regulations • Released December 12, 2008 for comment • Further specify Code of Conduct and disclosure requirements. • Largely conform to the Ph. RMA guidelines • DPH is waiting to see if further budget cuts are made before proceeding with the prescriber education program.

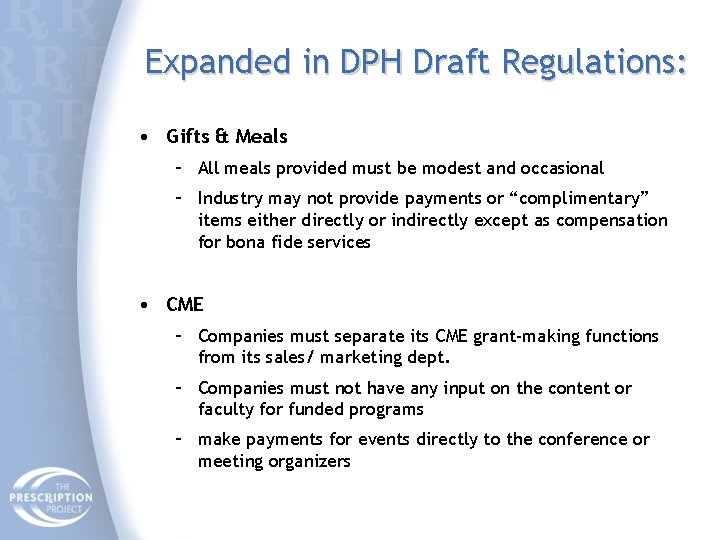
Expanded in DPH Draft Regulations: • Gifts & Meals – All meals provided must be modest and occasional – Industry may not provide payments or “complimentary” items either directly or indirectly except as compensation for bona fide services • CME – Companies must separate its CME grant-making functions from its sales/ marketing dept. – Companies must not have any input on the content or faculty for funded programs – make payments for events directly to the conference or meeting organizers

Expanded in DPH Draft Regulations: • Payments for Services (may be provided if: ) – the compensation is subject to a written contract – the compensation is fair market value – There is a legitimate need for the service – There is a connection between the competence/expertise of the consultant and the purpose of the arrangement – Only the number of consultants needed are retained – The company retains records about the arrangement and makes appropriate use of the services provided – The venue and circumstances of any meeting are appropriate

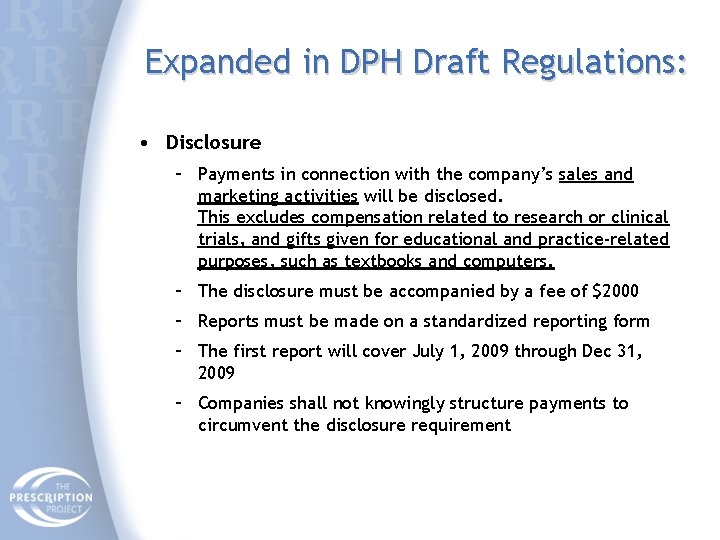
Expanded in DPH Draft Regulations: • Disclosure – Payments in connection with the company’s sales and marketing activities will be disclosed. This excludes compensation related to research or clinical trials, and gifts given for educational and practice-related purposes, such as textbooks and computers. – The disclosure must be accompanied by a fee of $2000 – Reports must be made on a standardized reporting form – The first report will cover July 1, 2009 through Dec 31, 2009 – Companies shall not knowingly structure payments to circumvent the disclosure requirement

Expanded in DPH Draft Regulations: • Other Disclosures – Industry must require speakers and commercial consultants to disclose their roles as such if they are also members of formulary/clinical guideline committees. • Use of Prescribing Information – All companies that use prescriber-identifiable prescribing information for marketing must: • Maintain the confidentiality of the data • Develop policies re: the use of the data, educate employees agents about the policies and identify disciplinary actions for violations • Comply with requests of providers to not provide his/her data to sales reps

Issues being discussed • General business environment • Payments related to research • Small gifts • Gifts with educational value • Industry support of CME, professional meetings, and events

General Business Environment • Concern: The C&Q measures will drive business from the state • Response: Since Minnesota’s gift ban and disclosure legislation passed in the early 1990 s, pharmaceutical industry jobs there grew by over 36%, compared with 19. 7% nationwide. • Disclosure of all relationships will allow the state and the public to understand the financial relationships between physicians and companies.

Payments Related to Research • Concern: disclosing payments made to researchers will harm a company’s competitive edge. • Response: The information being disclosed is not private. Pharmaceutical companies and providers already disclose information about clinical trials to the FDA and NIH. The FDA publishes this information on a public database.

Small Gifts and Educational Gifts • Concern: Banning small gifts will harm the relationship between industry and doctors, and will harm businesses that manufacture these gift-products. • Response: The power of small, repeated gifts to influence decision-making has been extensively documented. • Concern: Banning educational gifts does a disservice to patients and prevents industry from sharing information. • Response: Educational gifts are still part of marketing. The law does not impede information sharing. Sales representatives can continue to visit doctor’s offices and provide information about their products.

One appeal to the MA House

Industry Support of CME & events • Concern: Prohibiting industry from providing direct sponsorship to CME or from directly providing meals at professional meetings and events will drive symposia from the state. Caterers claim they will lose $40 million. • Response: ACCME regulations and Ph. RMA guidelines already largely conform with the C&Q law. The Minnesota Convention Center Bureau say they have not seen any dip in convention attendance since enactment of MN’s gift ban and disclosure legislation in 1993.

Thank you Questions, discussion? Allan Coukell, Director of Policy Tel: 617 -275 -2870 acoukell@communitycatalyst. org www. prescriptionproject. org
 National policy and legislation
National policy and legislation Role of rrrlf in modernization of libraries
Role of rrrlf in modernization of libraries Aviation legislation module 10 ppt
Aviation legislation module 10 ppt Examples of informal amendments
Examples of informal amendments Legislation
Legislation Health and safety legislation
Health and safety legislation Legislation related to maternity benefits
Legislation related to maternity benefits Loler 1998 legislation
Loler 1998 legislation Square deal
Square deal Environmental legislation in construction
Environmental legislation in construction Hierarchy of legislation
Hierarchy of legislation Legislation vs regulation
Legislation vs regulation Iso legislation update
Iso legislation update Hasawa legislation
Hasawa legislation Recommendation of drug enquiry committee
Recommendation of drug enquiry committee Tourism legislation and professinal ethics
Tourism legislation and professinal ethics Delegated legislation meaning
Delegated legislation meaning Legis meaning
Legis meaning Veterinary waste management
Veterinary waste management Traçabilité des soins infirmiers législation
Traçabilité des soins infirmiers législation Eu audit legislation
Eu audit legislation Thailand former name
Thailand former name Undoweled
Undoweled Types of legislation
Types of legislation Ict ethics and legislation
Ict ethics and legislation Closed loop geothermal well massachusetts
Closed loop geothermal well massachusetts Massachusetts office of travel & tourism
Massachusetts office of travel & tourism















































