IDE Decision Making Owen Faris Ph D Clinical









- Slides: 10

IDE Decision Making Owen Faris, Ph. D. Clinical Trials Director Office of Device Evaluation Center for Devices and Radiological Health

Some History: FY 14 -15 Clinical Trial Strategic Goal Improve the efficiency, consistency, and predictability of the IDE process to reduce the time and number of cycles needed to reach appropriate IDE full approval for medical devices, in general, and for devices of public health importance, in particular. 2

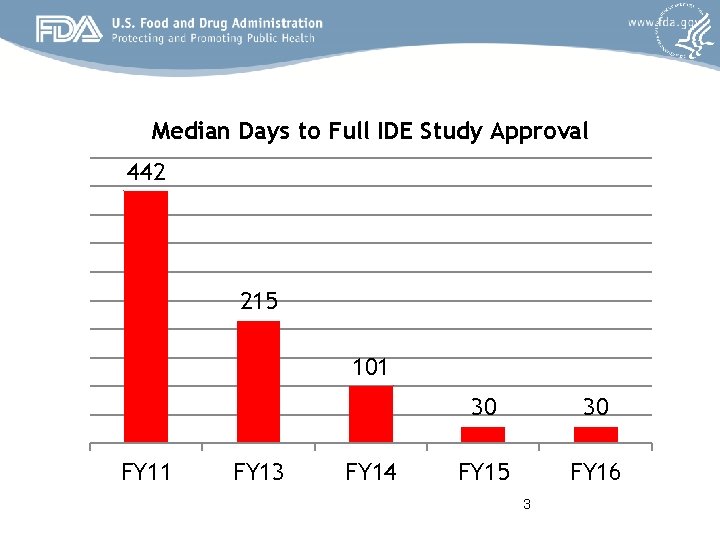
Median Days to Full IDE Study Approval 442 215 101 FY 13 FY 14 30 30 FY 15 FY 16 3

What has CDRH been doing? • Established Clinical Trials Program with visibility and oversight of ODE decisions • Established SOP for CT Program involvement and review of certain IDE decisions. Focus on: • Ensuring CDRH is “in the right place” • Ensuring flexibility is applied where appropriate • Increased communication with sponsors • Worked to establish a culture of improved collaboration and solution development with sponsors 4

Staff Education • FDA staff generally highly educated and engaged, but most have no first hand clinical trial experience • Education can help in – Understanding the impact of deficiencies and which “asks” are bigger than others – Understanding what other stakeholders and mitigations are in place in the clinical study to protect subjects 5

Staff education • In 2015 – 400+ premarket review staff participated in small group, half day clinical trial ecosystem training – ~125 staff participated in Experiential Learning Program visits to sponsor to learn about clinical trials • In 2016 – Plan to send ~125 staff on clinical trials ELP 6

Benefits and Risks • The most difficult IDE decisions rest on determining the level of risk to accept, considering: – The “point estimate” for our concern and the uncertainty – The seriousness of the potential risk for subjects – The stage of study – The potential benefits of the technology for subjects in the study and future patients, including consideration of alternatives – The potential consequences requiring more testing prior to IDE approval in terms of delaying 7 availability of promising technologies

Submission Quality • Many IDE submissions fail to “tell the sponsor’s story” • Many others fail to provide basic information needed to support FDA’s IDE review • Interaction with sponsor during IDE review can help resolve minor issues, but improvements in submission quality are a critical component as well 8

Types of questions that relate to submission quality • Describe device components and materials • Describe principle of operation and key characteristics • Clarify version of device tested compared to version for clinical study • Clarify what testing was done with rationale • Provide adequate description of test conditions, success criteria, and results 9

Clinical Trials Program: 2016 Plans • Continued monitoring of performance for IDEs in general and EFS IDEs • Finalize Benefit-Risk Guidance for IDEs • Submission quality improvements tools for external stakeholders • Active involvement in all three of CDRH’s 2016 -17 Strategic Priorities – Establish a National Evaluation System for medical devices and develop methods to better access and use real-world evidence – Partner with patients 10 – Promote a culture of quality and organizational excellence