Hospital Formularies DecisionMaking Process a CADTH Environmental Scan












- Slides: 17

Hospital Formularies Decision-Making Process a CADTH Environmental Scan KATHLEEN KULYK LIAISON OFFICER – SASKATCHEWAN ON BEHALF OF SIRJANA PANT 2015 CADTH SYMPOSIUM APRIL 13, 2015

Background v Pharmacy and Therapeutics Committees function at various levels to make formulary decisions v Independent process resulting in variations in formularies v More collaboration in recent years to venhance continuity of care, vensure equality of services, and vimprove the ability to align the hospital formulary with the publicly funded provincial outpatient drug program 1

Introduction Survey-based Environmental Scan - Level of awareness and use of CADTH products and services at Canadian health authorities and hospitals. - Identify potential opportunities for CADTH to support formulary review processes at Canadian health authorities and hospitals - Explore the level of collaboration between these health authorities and hospitals and the public drug plans when formulary decisions are being made. Pant S, Sherwood V, and Chelak K. Hospital Formularies Decision-Making Process. Ottawa: Canadian Agency for Drugs and Technologies in Health; 2015. www. cadth. ca 2

Method • 23 surveyed, responses received from 20 (June - October , 2014) • Currently involved in a health authority or hospital formulary decisionmaking process (except Quebec) – Total 19 respondents in findings • Representation from each province and territory. • Representation from both urban and rural settings in all the provinces (except Nova Scotia) • All respondents from the territories represented institutions servicing remote locations. • Two respondents were located in urban centres but provided support for remote locations (Nunavut and rural Ontario) via telepharmacy 3

Survey Questions on Collaboration 1. Describe any ongoing initiatives your institution may be initiating or exploring to better support your drug review process? 2. How do provincial drug plan formulary decisions influence your drug formulary decisions? 3. Are there any formal policies or informal processes in place and/or any initiatives underway to align with provincial drug plan recommendations? 4. 4 What challenges have been identified in your jurisdiction to align hospital formularies with provincial drug plan formularies?

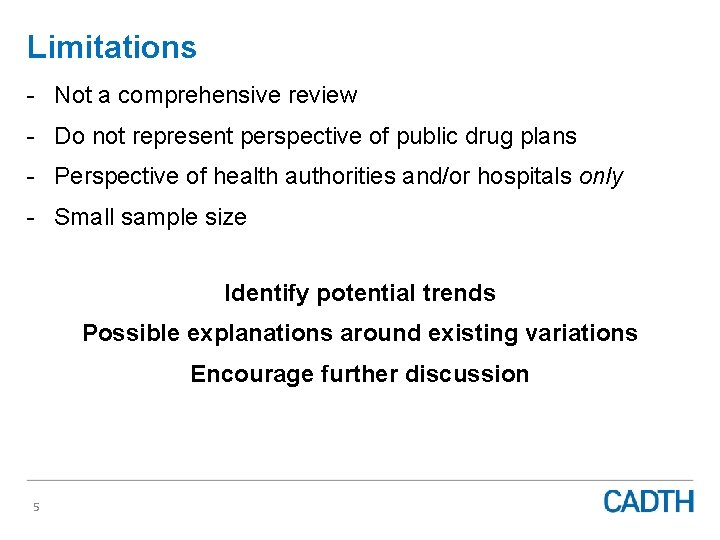
Limitations - Not a comprehensive review - Do not represent perspective of public drug plans - Perspective of health authorities and/or hospitals only - Small sample size Identify potential trends Possible explanations around existing variations Encourage further discussion 5

Findings 6

Level of Collaboration between 1. Hospitals and HAs in a province/territory 2. Public Drug Plan, and HAs and Hospitals in a province/ territory 7

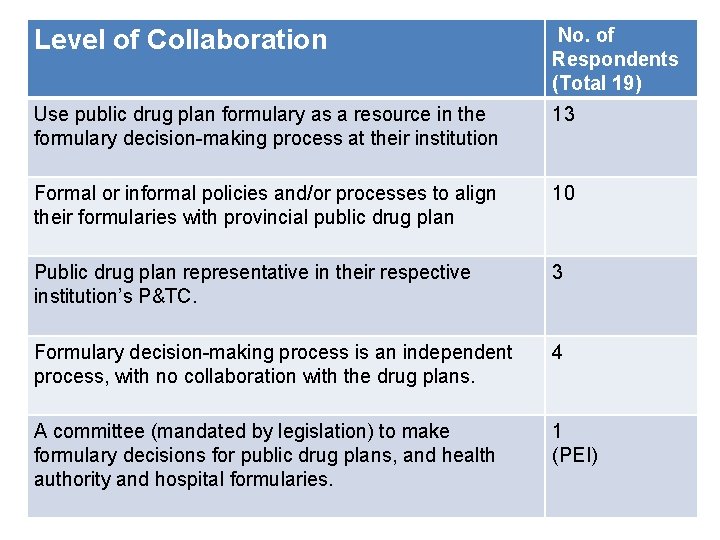
Level of Collaboration No. of Respondents (Total 19) Use public drug plan formulary as a resource therespondents, 13 Out of thein 19 formulary decision-making process at their institution v. Formal or informal policies and/or processes to align 10 v recommendations. their formularies with provincial public drug plan Public drug plan representative in their respective institution’s P&TC. 3 Formulary decision-making process is an independent process, with no collaboration with the drug plans. 4 A committee (mandated by legislation) to make formulary decisions for public drug plans, and health authority and hospital formularies. 1 (PEI) 8

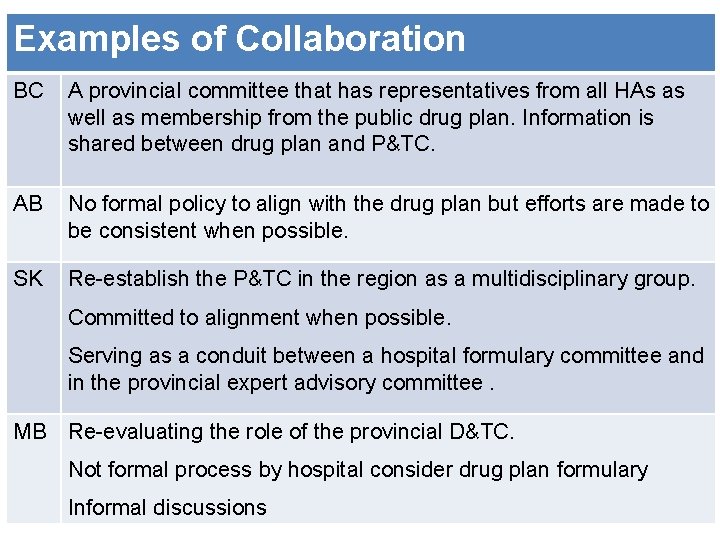
Examples of Collaboration BC A provincial committee that has representatives from all HAs as well as membership from the public drug plan. Information is shared between drug plan and P&TC. AB No formal policy to align with the drug plan but efforts are made to be consistent when possible. SK Re-establish the P&TC in the region as a multidisciplinary group. Committed to alignment when possible. Serving as a conduit between a hospital formulary committee and in the provincial expert advisory committee. MB Re-evaluating the role of the provincial D&TC. Not formal process by hospital consider drug plan formulary 9 Informal discussions

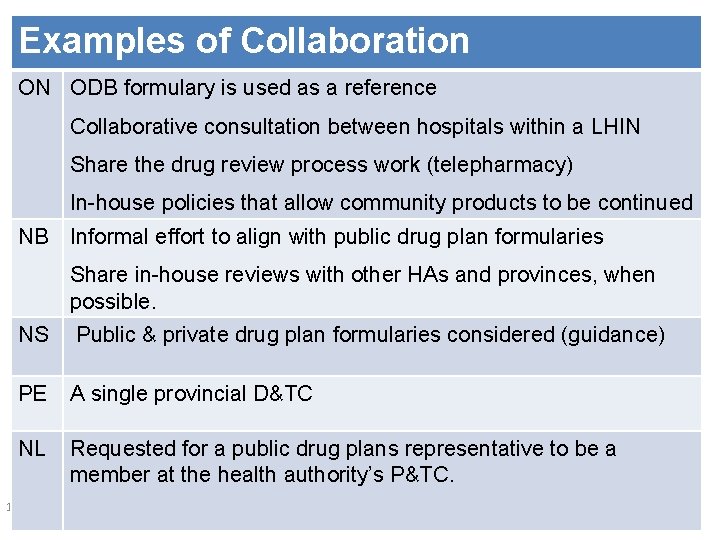
Examples of Collaboration ON ODB formulary is used as a reference Collaborative consultation between hospitals within a LHIN Share the drug review process work (telepharmacy) In-house policies that allow community products to be continued NB Informal effort to align with public drug plan formularies Share in-house reviews with other HAs and provinces, when possible. NS Public & private drug plan formularies considered (guidance) PE A single provincial D&TC NL Requested for a public drug plans representative to be a member at the health authority’s P&TC. 10

Challenges in Aligning Hospital Formularies With Provincial Drug Plan Formularies

Challenges v Role in the Health Care System v. Perception of public drug plans solely as the funders v. Factors Affecting the Overall Cost of Drug vcost of managing an illness and hospital contract cost v. Decision-Making Process v Factors influencing decision making. v Limit hospital formulary listings to guide best practices. v Viewpoints on who should influence decision making 12

Challenges v Implementation and Resources v Implementation of new medications, Inventory issues and aligning with federal and provincial plan. v. Product Type v. Different therapeutic considerations; access to emerging treatment (tertiary care centers); hospital formulary more restrictive in general. v Additional Issues v. Lack of resources to support a single equitable process v. Variation in local implementation 13

Conclusion v Various ongoing (formal/informal) efforts vestablishing a single committee to make formulary decisions for both public drug plans and hospitals in a province, vestablishing a single provincial hospital formulary, vestablishing provincial-level working groups or advisory committees, vre-evaluating the role of the P&TC, and/or vsharing drug reviews between institutions. 14

Conclusion v Hospitals and HAs consider public drug plan coverage. v Challenges in aligning with the public drug plan due to differences in v the roles of these institutions in the health care system, v factors affecting the overall cost of adding a drug to a formulary, v decision-making processes, v implementation and resources issues, v product types. 15

16