Hormonal Contraceptives and Risk of HIV Acquisition What




- Slides: 11

Hormonal Contraceptives and Risk of HIV Acquisition: What We Know • 30 years of epidemiologic and laboratory studies have tried to determine whethere is truly increased risk of HIV acquisition associated with use of hormonal contraception. • Some studies showed that progestin-only injectables, particularly the intramuscular injectable depot medroxyprogesterone acetate (DMPA-IM), were linked to increased HIV risk, but other studies did not show this result. – In meta-analyses, the magnitude of increased HIV risk was approximately 40 -50% (i. e. , hazard ratios of 1. 4 -1. 5) Session II supplement, Slide 1

Evidence for Contraceptive and HIV Outcomes (ECHO) Trial • ECHO was a multicentre, open-label, randomised clinical trial comparing HIV incidence and contraceptive benefits in women living in areas of high HIV incidence and using one of three highly-effective, licensed contraceptive methods: – intramuscularly-delivered depot medroxyprogesterone acetate (DMPA-IM) – a copper intrauterine device (IUD) – and a levonorgestrel (LNG) implant (Jadelle) • Eliminates bias by using randomization for assigning contraceptive methods • 9 study sites in South Africa, one each in Kenya, Swaziland Zambia Session II supplement, Slide 2

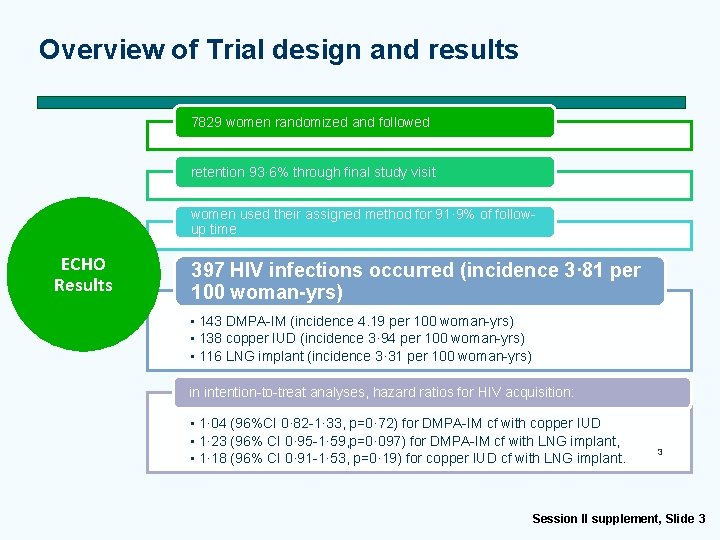
Overview of Trial design and results 7829 women randomized and followed retention 93· 6% through final study visit women used their assigned method for 91· 9% of followup time ECHO Results 397 HIV infections occurred (incidence 3· 81 per 100 woman-yrs) • 143 DMPA-IM (incidence 4. 19 per 100 woman-yrs) • 138 copper IUD (incidence 3· 94 per 100 woman-yrs) • 116 LNG implant (incidence 3· 31 per 100 woman-yrs) in intention-to-treat analyses, hazard ratios for HIV acquisition: • 1· 04 (96%CI 0· 82 -1· 33, p=0· 72) for DMPA-IM cf with copper IUD • 1· 23 (96% CI 0· 95 -1· 59, p=0· 097) for DMPA-IM cf with LNG implant, • 1· 18 (96% CI 0· 91 -1· 53, p=0· 19) for copper IUD cf with LNG implant. 3 Session II supplement, Slide 3

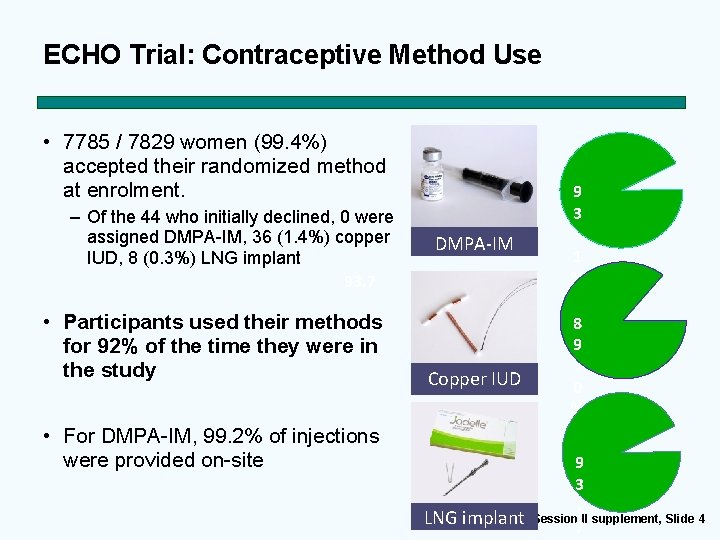
ECHO Trial: Contraceptive Method Use • 7785 / 7829 women (99. 4%) accepted their randomized method at enrolment. – Of the 44 who initially declined, 0 were assigned DMPA-IM, 36 (1. 4%) copper IUD, 8 (0. 3%) LNG implant 93. 7 • Participants used their methods for 92% of the time they were in the study • For DMPA-IM, 99. 2% of injections were provided on-site DMPA-IM Copper IUD 9 3. 1 % 8 9. 0 % 9 3. Session II supplement, Slide 4 LNG implant 7

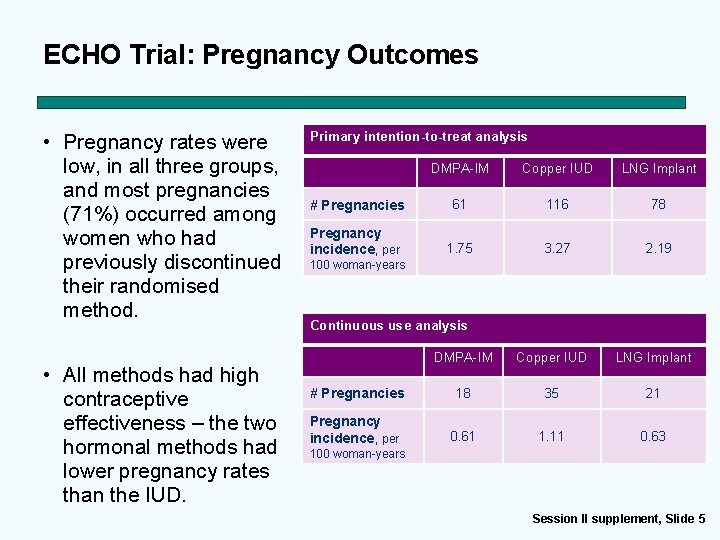
ECHO Trial: Pregnancy Outcomes • Pregnancy rates were low, in all three groups, and most pregnancies (71%) occurred among women who had previously discontinued their randomised method. • All methods had high contraceptive effectiveness – the two hormonal methods had lower pregnancy rates than the IUD. Primary intention-to-treat analysis DMPA-IM Copper IUD LNG Implant # Pregnancies 61 116 78 Pregnancy incidence, per 1. 75 3. 27 2. 19 100 woman-years Continuous use analysis DMPA-IM Copper IUD LNG Implant # Pregnancies 18 35 21 Pregnancy incidence, per 0. 61 1. 11 0. 63 100 woman-years Session II supplement, Slide 5

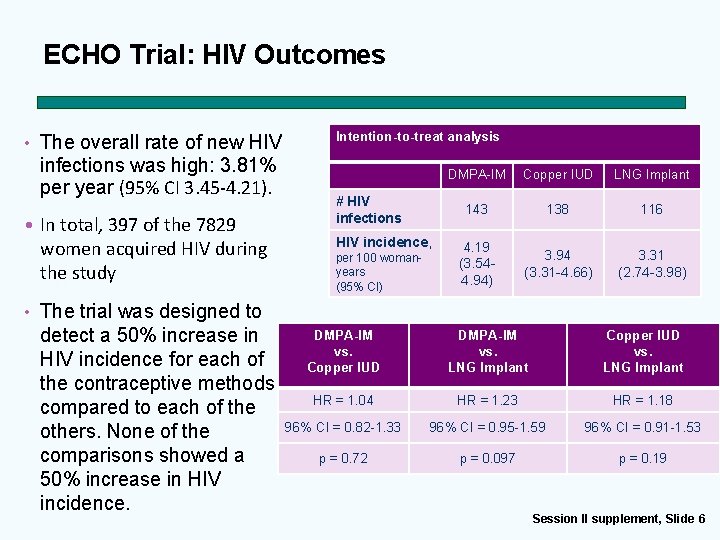
ECHO Trial: HIV Outcomes • The overall rate of new HIV infections was high: 3. 81% per year (95% CI 3. 45 -4. 21). • In total, 397 of the 7829 women acquired HIV during the study Intention-to-treat analysis # HIV infections HIV incidence, per 100 womanyears (95% CI) DMPA-IM Copper IUD LNG Implant 143 138 116 4. 19 (3. 544. 94) 3. 94 (3. 31 -4. 66) 3. 31 (2. 74 -3. 98) • The trial was designed to detect a 50% increase in HIV incidence for each of the contraceptive methods compared to each of the others. None of the comparisons showed a 50% increase in HIV incidence. DMPA-IM vs. Copper IUD DMPA-IM vs. LNG Implant Copper IUD vs. LNG Implant HR = 1. 04 HR = 1. 23 HR = 1. 18 96% CI = 0. 82 -1. 33 96% CI = 0. 95 -1. 59 96% CI = 0. 91 -1. 53 p = 0. 72 p = 0. 097 p = 0. 19 Session II supplement, Slide 6

ECHO Trial: Key Findings • The multi-country randomised trial measured HIV incidence among African women assigned to one of three highly-effective contraceptive methods. • Contraceptive findings: – All methods were acceptable and had very high rate of continuation. – All methods were highly effective and were associated with very low pregnancy rates. – All methods were safe – few serious adverse events. • HIV Findings: – No significant difference in HIV acquisition risk among contraceptive methods. – HIV incidence was high among women using all three contraceptive methods and slightly higher among younger women. – For individual women at very high HIV risk, we acknowledge that even a relatively small effect might be important in contraceptive and HIV prevention decision-making Session II supplement, Slide 7

Programmatic Implications of ECHO Findings • Family Planning – These results underscore the importance of continued and increased access to these three contraceptive methods, as well as expanded contraceptive choices, complemented by high-quality HIV and STI prevention services. – This trial demonstrates that delivery of high-quality services to support the use of the copper IUD and the LNG implant across multiple African settings is possible with appropriate investment in training, assurance of provider clinical competency, adequate human resources for counselling and management of side effects, and necessary logistical support including management of commodities. – The results cannot be generalized to other contraceptive methods not included in the study (e. g. , NET-En, DMPA-SC, hormone-containing IUDs, etc. ) Session II supplement, Slide 8

Programmatic Implications of ECHO Findings • HIV – In spite of an individualized HIV prevention package provided to all participants throughout follow-up and country-wide HIV treatment and prevention programmes, HIV incidence was alarmingly high in this population throughout the course of the trial and STI prevalence at baseline was also very high. – The results strongly emphasize the need for more aggressive HIV and STI prevention and management efforts for African women, including HIV preexposure prophylaxis (Pr. EP) and HIV prevention integrated with contraceptive services. Session II supplement, Slide 9

ECHO Trial and Family Planning: Key Points • WHO will provide updated Medical Eligibility Criteria guidance on hormonal contraception for women at high risk of HIV in September 2019. • In the interim the WHO 2017 MEC Guidance for women at high risk of HIV should continue to be followed. Women at high risk of HIV who consider progestin-only injectables should not be restricted from use and should: – Be advised about the potential increased risk of HIV acquisition because of risky situations and acts. – Receive HIV prevention information – Be advised that dual method use is the best option to prevent both STI/HIV acquisition and unintended pregnancy – Have access to a wide range of contraceptive methods to make a free and informed choice of the method that best fits their needs/situation (including their HIV risk) Session II supplement, Slide 10

Additional Resources on the ECHO Trial • For further information on the ECHO Trial and its results please go to the following sources: – The official site of the ECHO Consortium http: //echo-consortium. com/ • This site contains a variety of resources for understanding the trial, its results and the way forward, including press releases, official statements, published articles, slide decks and – The presentation of the results in South Africa https: //www. youtube. com/watch? v=g. Ugn. La 24 GBc&feature=youtu. be – The WHO page for the ECHO Trial https: //www. who. int/reproductivehealth/hc-hiv/en/ • This site provides a statement on the trial and its results in English, French and Spanish, as well as links to additional materials about the trial, its results, and going forward Session II supplement, Slide 11