Homeroom Warm Up 101518 Write 1 paragraph to




















- Slides: 29

Homeroom Warm Up 10/15/18 Write 1 paragraph to summarize how you spent your fall break?

Intervention Warm Up 10/15/18 Write 1 paragraph to summarize how you spent your fall break?

Science Warm Up 10/15/18 Which of these statements describes a chemical property of an object? A. The object is white in color. B. The object has a powdery texture. C. The object’s density is 2. 11 g/cm 3 D. The object reacts with acid to form water.

Take a look at the picture below and complete the following sentences with your own responses 1. This makes me think of…. 2. I wonder…. 3. I have some questions…. 4. This makes me want to….

Introduction to Chemical Reactions 7. PS 1. 4 Analyze and interpret chemical reactions to determine if the total number of atoms in the reactants and products support the Law of Conservation of Mass.

Objective I can complete a graphic organizer to identify signs that a chemical reaction has taken place.

Vocabulary Terms 1. chemical reaction 2. chemical formula 3. exothermic reaction 4. chemical equation 5. endothermic reaction 6. reactant 7. law of conservation of energy 8. product 9. law of conservation of mass

Main Ideas Chemical Reactions are represented by Chemical Equations are balanced to show the same number of atoms of each element on each side. The Law of Conservation of Mass says that atoms won’t be created or destroyed in a chemical reaction. That is why you have to balance chemical equations!

Observing chemical Reactions Everything around us is made of atoms. These atoms are constantly interacting with each other. We call those interactions chemical reactions, and they are happening EVERYWHERE. (c) 2012 -Present Real Ms. Frizzle

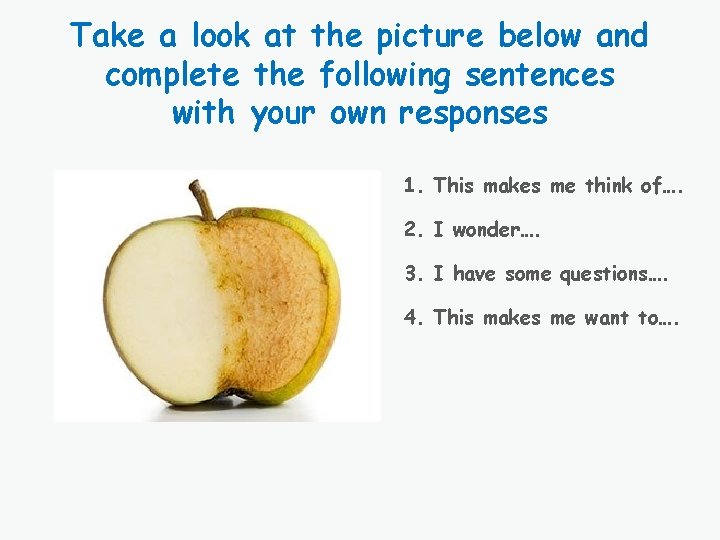
Chemical Reactions are Everywhere Cooking Respiration

Chemical Reactions are Everywhere Hair Dye Auto Fuel

How do you know when a chemical reaction takes place? Color Change Precipitate Formation

How do you know when a chemical reaction takes place? Gas Formation Odor

How do you know when a chemical reaction takes place? Temperature Change in Acidity

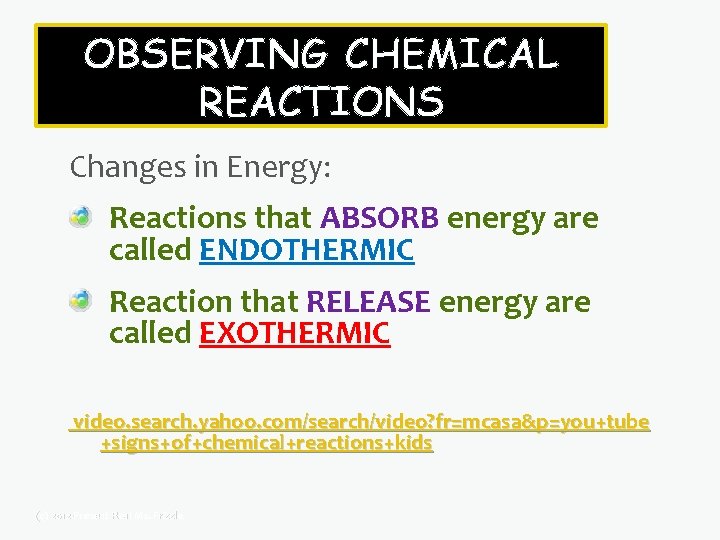
OBSERVING CHEMICAL REACTIONS Changes in Energy: Some reactions ABSORB energy Other reactions RELEASE energy You can usually tell if something is absorbing or releasing energy because there is a CHANGE IN TEMPERATURE. (c) 2012 -Present Real Ms. Frizzle

OBSERVING CHEMICAL REACTIONS Changes in Energy: Reactions that ABSORB energy are called ENDOTHERMIC Reaction that RELEASE energy are called EXOTHERMIC video. search. yahoo. com/search/video? fr=mcasa&p=you+tube +signs+of+chemical+reactions+kids (c) 2012 -Present Real Ms. Frizzle

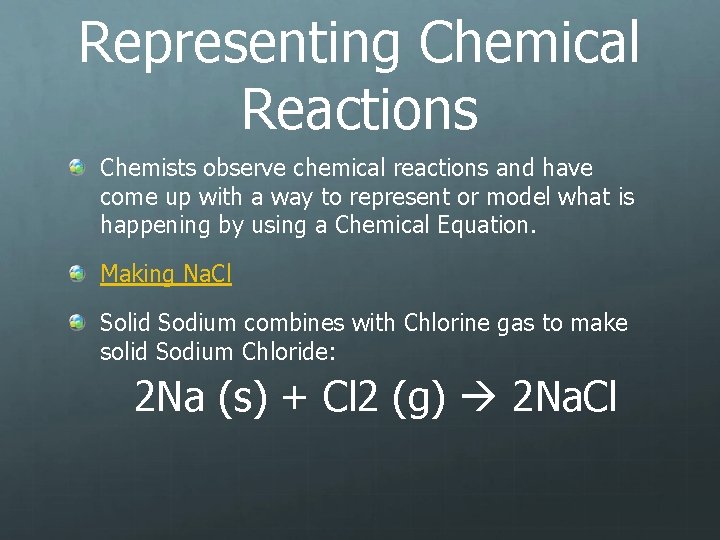
Representing Chemical Reactions Chemists observe chemical reactions and have come up with a way to represent or model what is happening by using a Chemical Equation. Making Na. Cl Solid Sodium combines with Chlorine gas to make solid Sodium Chloride: 2 Na (s) + Cl 2 (g) 2 Na. Cl

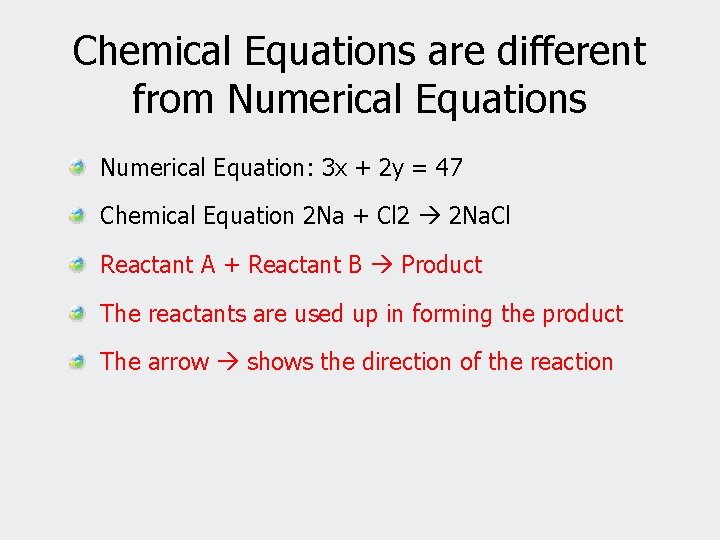
Chemical Equations are different from Numerical Equations Numerical Equation: 3 x + 2 y = 47 Chemical Equation 2 Na + Cl 2 2 Na. Cl Reactant A + Reactant B Product The reactants are used up in forming the product The arrow shows the direction of the reaction

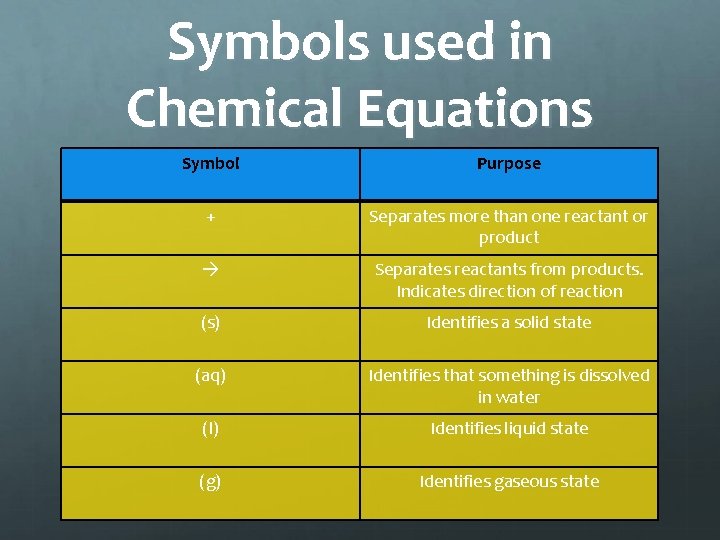
Symbols used in Chemical Equations Symbol Purpose + Separates more than one reactant or product Separates reactants from products. Indicates direction of reaction (s) Identifies a solid state (aq) Identifies that something is dissolved in water (l) Identifies liquid state (g) Identifies gaseous state

Law of Conservation of Mass In a chemical reaction, matter is neither created nor destroyed. Atoms won’t change their identity (e. g. a Carbon atom can’t become an Iron atom) This means that you have to have the same number of each type of atom on each side of the chemical equation. Conservation of Mass Video

Science Closure 10/15/18 Which of the following occurrences indicates that a chemical reaction has taken place? A. An odor is produced by burning a sugar cube. B. A puddle is produced by melting an ice cube. C. A loud noise is produced by crushing a can. D. A piece of glass is produced by breaking a bottle.

Balancing Equations After you write a chemical equation you have to balance it to make sure that the same number of atoms of each element are on each side. How would you balance this equation? Li + H 2 O H 2 + Li. OH

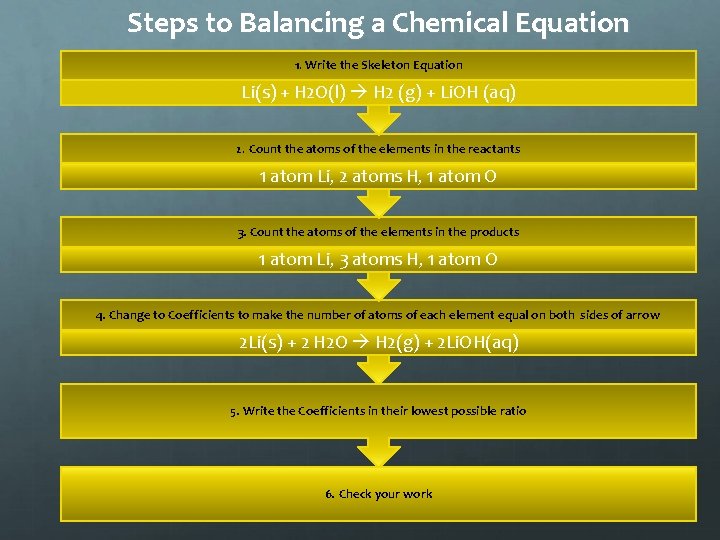
Steps to Balancing a Chemical Equation 1. Write the Skeleton Equation Li(s) + H 2 O(l) H 2 (g) + Li. OH (aq) 2. Count the atoms of the elements in the reactants 1 atom Li, 2 atoms H, 1 atom O 3. Count the atoms of the elements in the products 1 atom Li, 3 atoms H, 1 atom O 4. Change to Coefficients to make the number of atoms of each element equal on both sides of arrow 2 Li(s) + 2 H 2 O H 2(g) + 2 Li. OH(aq) 5. Write the Coefficients in their lowest possible ratio 6. Check your work

Another Example CH 4 (methane gas) + O 2 CO 2 + H 2 O Reactants Products # of Carbons = 1 # of Hydrogens = 4 # of Hydrogens = 2 # of Oxygens = 3 Total atoms = 7 Total atoms = 6 7 ≠ 6! Where did our atoms go?

Example Continued Change the Coefficients to make the number of atoms of each element equal Balance the Hydrogens: CH 4 + O 2 CO 2 + 2 H 2 O Balance the Oxygens: CH 4 + 2 O 2 CO 2 + 2 H 2 O

Example Continued CH 4 + 2 O 2 CO 2 + 2 H 2 O Are your coefficients in their simplest ratio? Count your atoms again to check your work: Reactants Products # of Carbons = 1 # of Hydrogens = 4 # of Oxygens = 4 Total atoms = 9

Try These! C 2 H 6 + O 2 CO 2 + H 2 O Fe 2 O 3 + H 2 SO 4 Fe 2(SO 4)3 + H 2 O Hint : balance the polyatomic ion first! Ca. Cl 2 + Ag. NO 3 Ag. Cl + Ca(NO 3)2 Think – Pair - Share

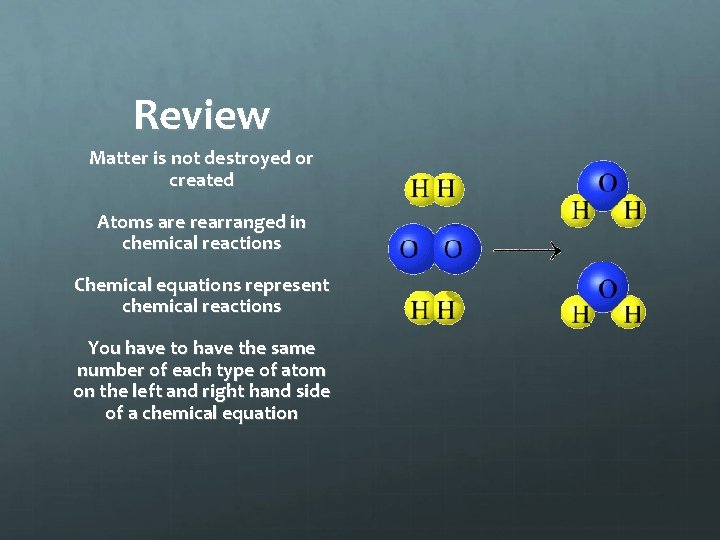
Review Matter is not destroyed or created Atoms are rearranged in chemical reactions Chemical equations represent chemical reactions You have to have the same number of each type of atom on the left and right hand side of a chemical equation

WARNING! Don’t mess with the insides of polyatomic ions – put a square around them, or label them as X – treat the WHOLE polyatomic ion as though it were an element! Don’t ever play around with subscripts (those little numbers that tell you how many atoms are in a molecule) e. g. C 6 H 22 O 11
 Ssds nj homeroom
Ssds nj homeroom West cabarrus high school open house
West cabarrus high school open house Homeroom expectations
Homeroom expectations Homeroom expectations
Homeroom expectations Homeroom.state.nj.us
Homeroom.state.nj.us Cache memory adalah
Cache memory adalah Analytical paragraph structure
Analytical paragraph structure Define expository paragraph with example
Define expository paragraph with example Th paragraphs
Th paragraphs Aec format
Aec format Opinion paragraphs
Opinion paragraphs Example of five sentence paragraph
Example of five sentence paragraph Two chunk paragraph
Two chunk paragraph The means of transport i like most
The means of transport i like most Theme statemtn
Theme statemtn Example of problem-solution paragraph
Example of problem-solution paragraph What is a tbear paragraph
What is a tbear paragraph Supporting an opinion
Supporting an opinion Peter writing technique
Peter writing technique P.e.e essay structure
P.e.e essay structure Writing in paragraph form
Writing in paragraph form What is the narrative paragraph
What is the narrative paragraph Example of jane schaffer paragraph
Example of jane schaffer paragraph Teal paragraph example
Teal paragraph example Tcea paragraph
Tcea paragraph How to right a counterclaim
How to right a counterclaim Paragraph hamburger
Paragraph hamburger Thesis statement examples for research papers
Thesis statement examples for research papers Ppe point proof explain
Ppe point proof explain Daily routine paragraph in spanish
Daily routine paragraph in spanish