GOOD MORNING Today we will Turn in your






- Slides: 17

GOOD MORNING! Today we will: Turn in your Periodic Table Article – Staple your researched article • Warm Up • Properties of Light Notes & Practice Problems – Wavelength, frequency, energy • HW: Prop of Light practice problems, Unit 3 vocab DUE Tues (10/6)

WARM UP: What will be different? • Explain what will be different about atomic masses on the periodic table in the future. • Provide 2 situations in which isotope abundances vary.

What is Light (electromagnetic radiation)? • Electromagnetic radiation (EMR) is energy in the form of electromagnetic waves • What are electro magnetic waves? Radiant energy that travels through space, as waves • Electromagnetic waves consist of particles with charge and magnetic properties

Properties of Light Waves Frequency (ν) = number of complete waves per second Units = Hertz (Hz) or inverse seconds (s 1 -) or (1/s) A complete wave is one full cycle. Amplitude Wavelength (λ)= The length of one complete wave cycle Units = Ǻ, nm, cm, m

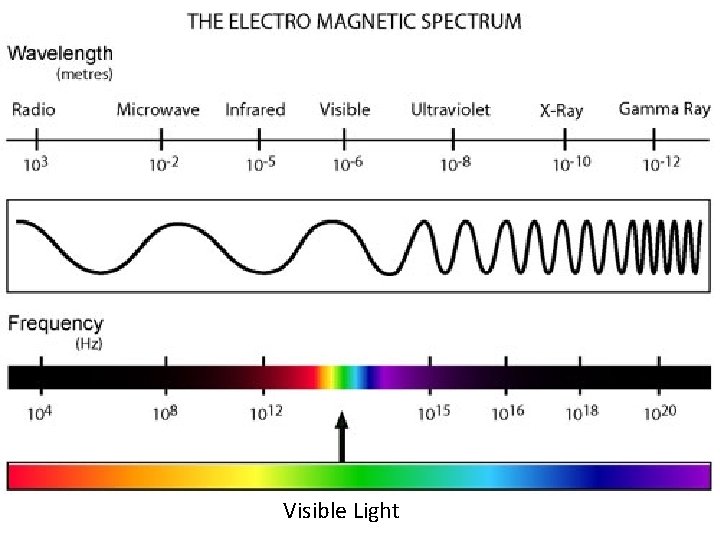
Properties of Electromagnetic Radiation Full Electromagnetic Spectrum Radio micro infrared visible ultraviolet x-rays gamma -5 10 -6 10 -8 10 -10 10 -12 λ (m): 100 0. 001 10 Freq (sec 1 -): Energy(J): Visible Light λ (nm): 700 600 500 400 1 -): Freq(sec Energy (J): http: //phet. colorado. edu/en/simulation/wave-on-a-string http: //phet. colorado. edu/en/simulation/radio-waves

Visible Light

What is light made of? Photons of light are “pieces” of energy or “particles” that behave as waves. This property is referred to as “wave-particle duality”. Thanks to me! Explain why a photon that appears violet has higher energy than a photon that appears green.

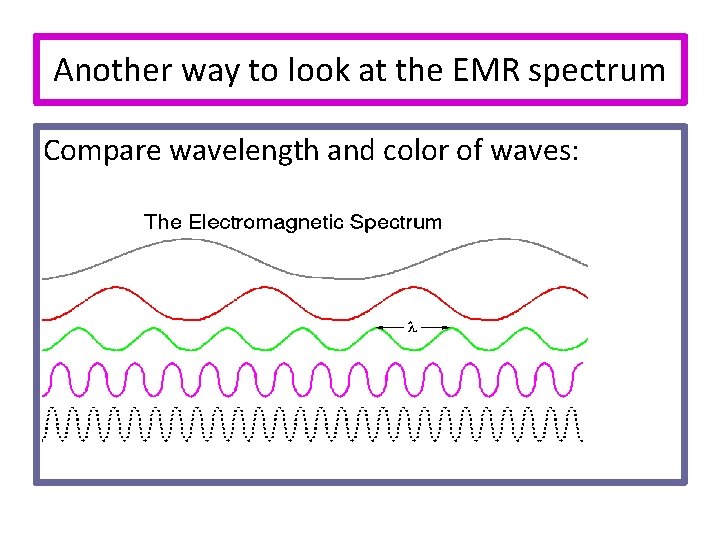
Another way to look at the EMR spectrum Compare wavelength and color of waves:

Important Relationships, Equations, and Constants Speed of Light = (freq) (wavelength) c = (f)(w) f = 1/s or s-1 c = 3. 0 x 108 m/sec w = m c = 3. 0 x 1010 cm/sec w = cm c = 3. 0 x 1017 nm/sec w = nm Energy = (Planck’s constant) (freq) E = (h)(f) f = 1/s or s-1 Planck’s constant (h) = 6. 63 x 10 -34 J∙s

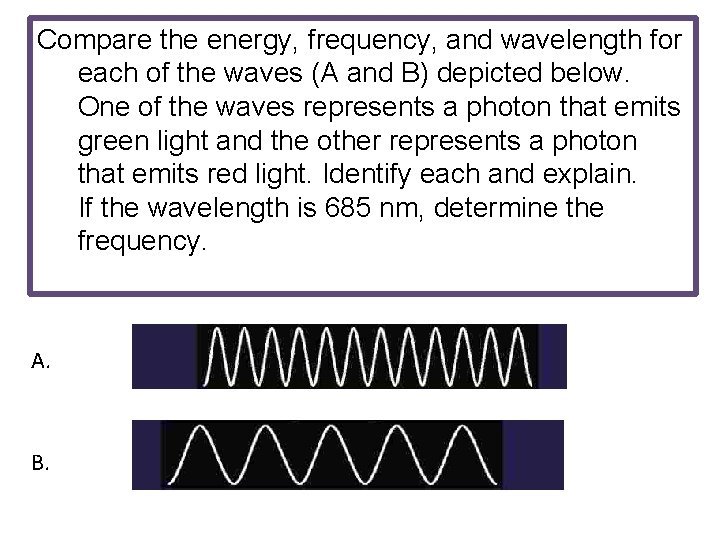
Compare the energy, frequency, and wavelength for each of the waves (A and B) depicted below. One of the waves represents a photon that emits green light and the other represents a photon that emits red light. Identify each and explain. If the wavelength is 685 nm, determine the frequency. A. B.

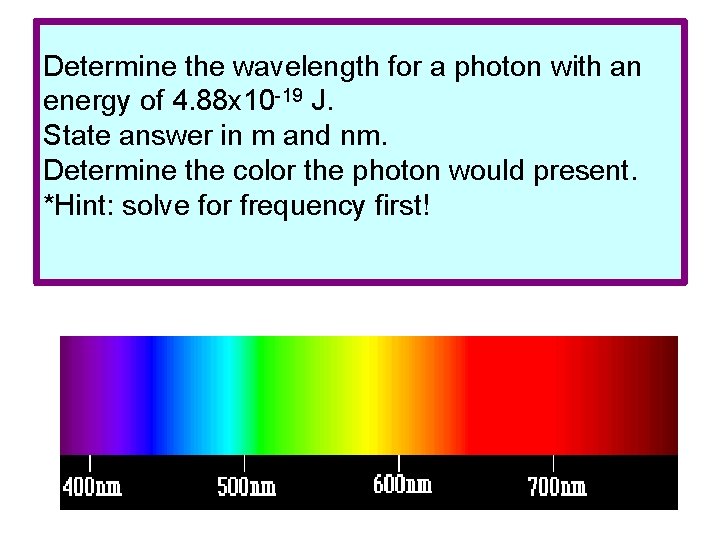
Determine the wavelength for a photon with an energy of 4. 88 x 10 -19 J. State answer in m and nm. Determine the color the photon would present. *Hint: solve for frequency first!

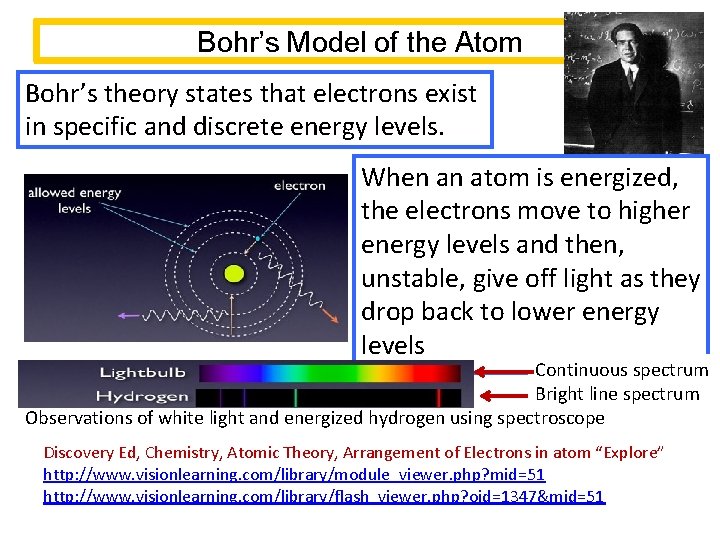
Bohr’s Model of the Atom Bohr’s theory states that electrons exist in specific and discrete energy levels. When an atom is energized, the electrons move to higher energy levels and then, unstable, give off light as they drop back to lower energy levels Continuous spectrum Bright line spectrum Observations of white light and energized hydrogen using spectroscope Discovery Ed, Chemistry, Atomic Theory, Arrangement of Electrons in atom “Explore” http: //www. visionlearning. com/library/module_viewer. php? mid=51 http: //www. visionlearning. com/library/flash_viewer. php? oid=1347&mid=51

Summary of Bohr’s Atom • Electrons exist in discrete, circular energy levels • Electrons cannot move continuously but only in precise steps or jumps from one level to another • The energy electrons absorb or release is “quantized” • When an atom absorbs energy, electrons move to higher energy levels – excited state – away from the nucleus • When an atom emits or releases energy, electrons move to lower energy levels – ground state – toward the nucleus • Different elements produce different emission spectra (set of lines of visible light called a bright line spectrum) • The lines in an emission spectrum are the result of electrons moving from higher energy levels to lower energy levels following an increase in energy • Each photon released represents a “quantum” of energy

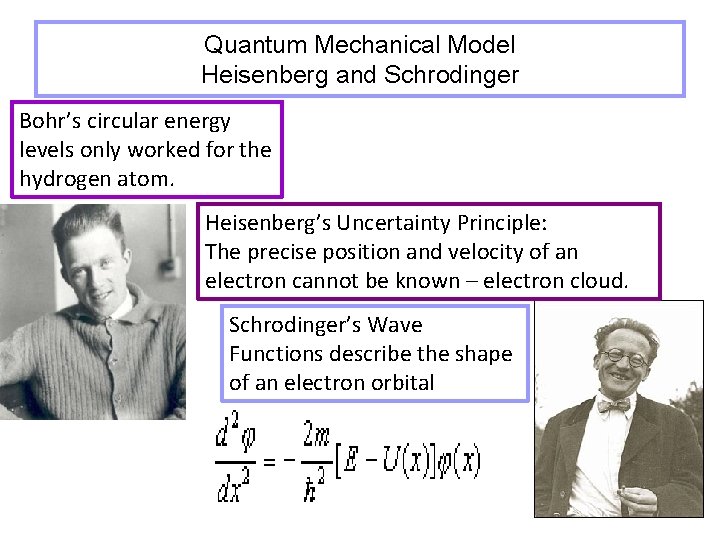
Quantum Mechanical Model Heisenberg and Schrodinger Bohr’s circular energy levels only worked for the hydrogen atom. Heisenberg’s Uncertainty Principle: The precise position and velocity of an electron cannot be known – electron cloud. Schrodinger’s Wave Functions describe the shape of an electron orbital

Then came Strider ….

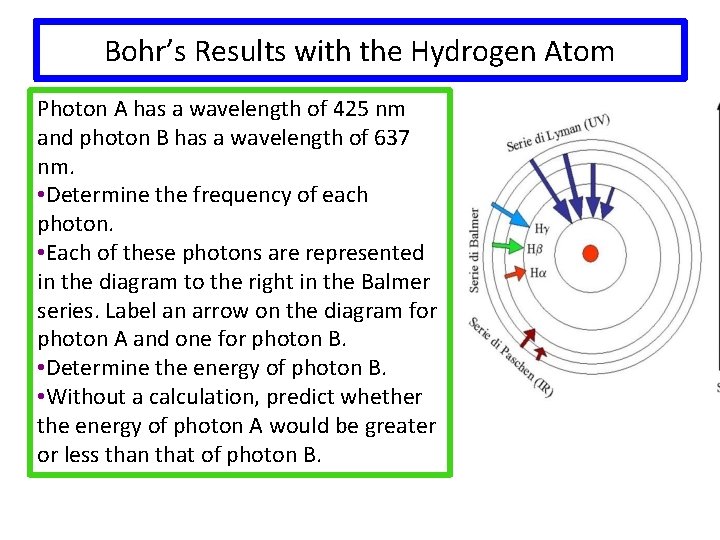
Bohr’s Results with the Hydrogen Atom Photon A has a wavelength of 425 nm and photon B has a wavelength of 637 nm. • Determine the frequency of each photon. • Each of these photons are represented in the diagram to the right in the Balmer series. Label an arrow on the diagram for photon A and one for photon B. • Determine the energy of photon B. • Without a calculation, predict whether the energy of photon A would be greater or less than that of photon B.

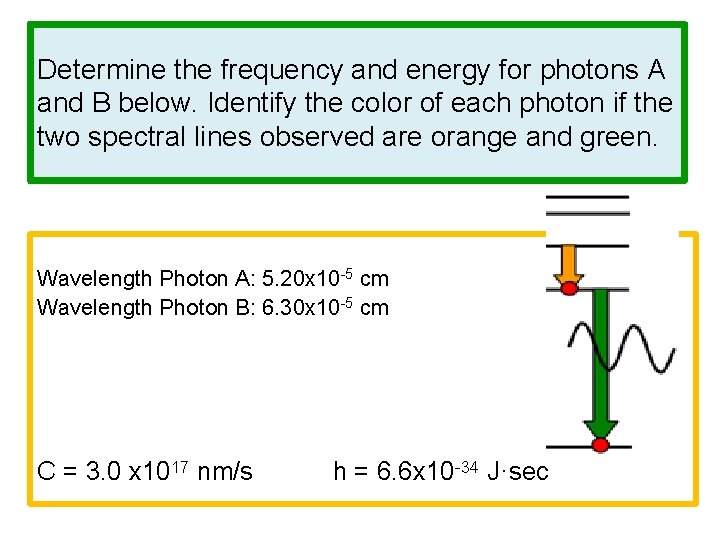
Determine the frequency and energy for photons A and B below. Identify the color of each photon if the two spectral lines observed are orange and green. Wavelength Photon A: 5. 20 x 10 -5 cm Wavelength Photon B: 6. 30 x 10 -5 cm C = 3. 0 x 1017 nm/s h = 6. 6 x 10 -34 J·sec
 Como te llamas in spanish
Como te llamas in spanish Good morning class images
Good morning class images Teacher good afternoon
Teacher good afternoon Good morning everyone vs everybody
Good morning everyone vs everybody Good morning nice to see you
Good morning nice to see you Hi hi good morning video
Hi hi good morning video Good afternon animado
Good afternon animado Good morning pupils
Good morning pupils Good morning my students
Good morning my students Good morning students how are you today
Good morning students how are you today Good morning students how are you today
Good morning students how are you today Good morning campers today's challenge is simple
Good morning campers today's challenge is simple Pronoun mad libs
Pronoun mad libs Another way to say good morning
Another way to say good morning Conversation good morning
Conversation good morning Good morning campers today's challenge is simple
Good morning campers today's challenge is simple Good morning challenge
Good morning challenge Hello good morning students
Hello good morning students
































