Executive Insight Clinical Evidence Communication Executive Insight AG



- Slides: 12

Executive Insight Clinical Evidence Communication Executive Insight AG. Company Snapshot Presentation.

Environmental trends have increased the internal and external demand for medical affairs contributions xxx Broadening and increasingly networked stakeholder base also for medical affairs (directly or indirectly), in particular adding regulatory and payers to the traditional KOL, HCP and society focus Raising stakeholders expectations on the quality of interactions within increasingly shorter time having shifted focus back to medical affairs field efforts and engagement – it’s relevance to many stakeholders is at par or even surpassed that of sales force activities Higher stakeholder demands on balanced value demonstration, requiring comprehensive evidence generation, collection and integration to support tailored communication on on unmet need, disease burden, clinical benefits as well as increasingly also economic benefits Medical affairs has become an essential hub connecting internal functions with external stakeholders, in particular for launch Executive Insight AG 2

Hence we believe in medical affairs strategic leadership in enabling solid stakeholder communication Executive Insight AG Align with other functions on wider mid-to long-term aims within a TA as well as crossfunctional strategic imperatives Ensure evidence is disseminated consistently to stakeholders across the organization following a common story Drive development of a body of evidence consisting of clinical trial data, RWE and existing research Identify and effectively communicate win-win areas, where external stakeholders and internal functions benefit from engagement 3

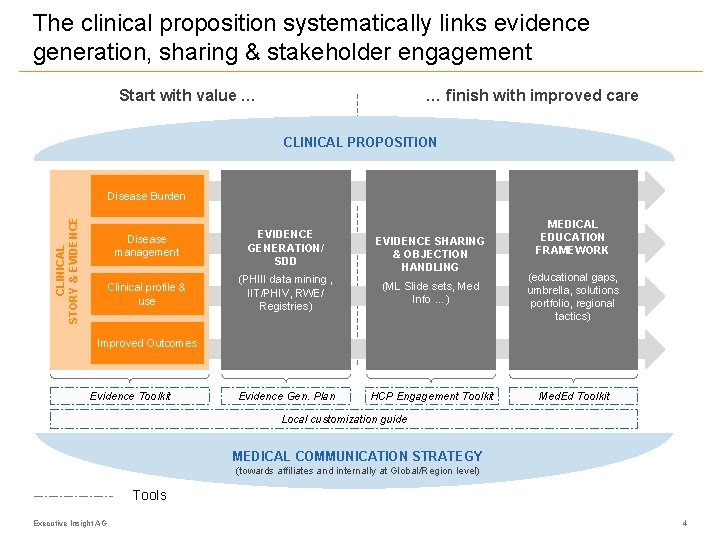
The clinical proposition systematically links evidence generation, sharing & stakeholder engagement Start with value … … finish with improved care CLINICAL PROPOSITION CLINICAL STORY & EVIDENCE Disease Burden Disease management EVIDENCE GENERATION/ SDD Clinical profile & use (PHIII data mining , IIT/PHIV, RWE/ Registries) EVIDENCE SHARING & OBJECTION HANDLING MEDICAL EDUCATION FRAMEWORK (ML Slide sets, Med Info …) (educational gaps, umbrella, solutions portfolio, regional tactics) HCP Engagement Toolkit Med. Ed Toolkit Improved Outcomes Evidence Toolkit Evidence Gen. Plan Local customization guide MEDICAL COMMUNICATION STRATEGY (towards affiliates and internally at Global/Region level) Tools Executive Insight AG 4

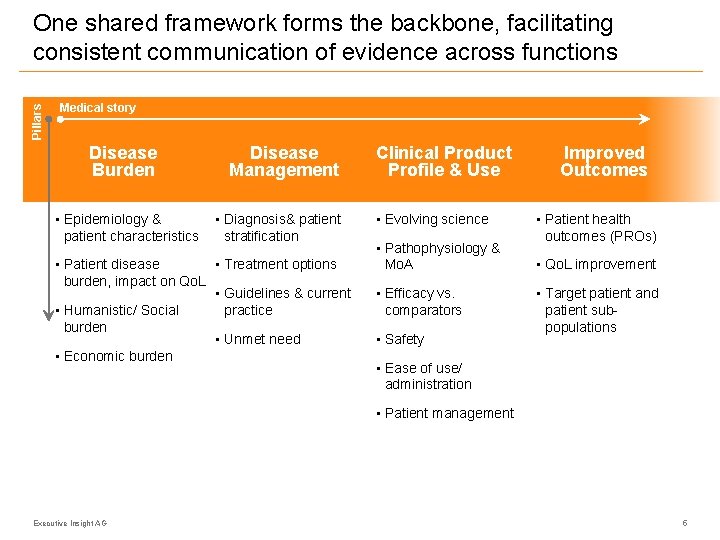
Pillars One shared framework forms the backbone, facilitating consistent communication of evidence across functions Medical story Disease Burden • Epidemiology & patient characteristics Disease Management • Diagnosis& patient stratification • Treatment options • Patient disease burden, impact on Qo. L • Guidelines & current practice • Humanistic/ Social burden • Unmet need • Economic burden Clinical Product Profile & Use • Evolving science • Pathophysiology & Mo. A • Efficacy vs. comparators • Safety Improved Outcomes • Patient health outcomes (PROs) • Qo. L improvement • Target patient and patient subpopulations • Ease of use/ administration • Patient management Executive Insight AG 5

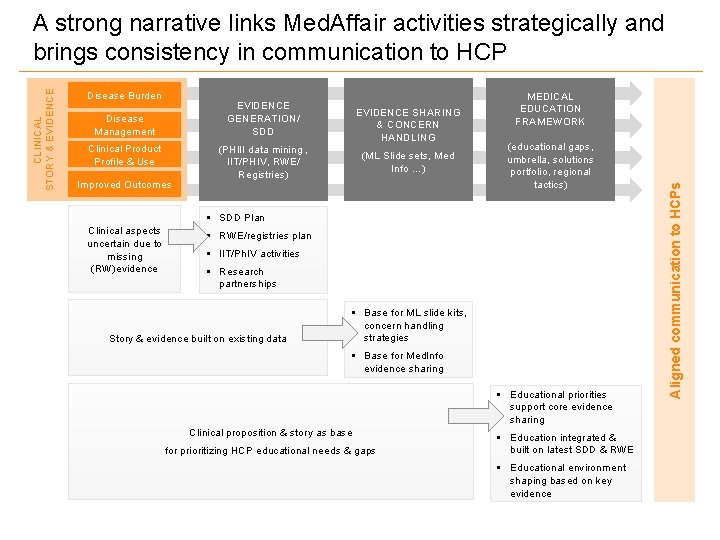
Disease Burden EVIDENCE GENERATION/ SDD Disease Management Clinical Product Profile & Use Improved Outcomes EVIDENCE SHARING & CONCERN HANDLING (PHIII data mining , IIT/PHIV, RWE/ Registries) (ML Slide sets, Med Info …) MEDICAL EDUCATION FRAMEWORK (educational gaps, umbrella, solutions portfolio, regional tactics) § SDD Plan Clinical aspects uncertain due to missing (RW)evidence § RWE/registries plan § IIT/Ph. IV activities § Research partnerships Story & evidence built on existing data § Base for ML slide kits, concern handling strategies § Base for Med. Info evidence sharing § Educational priorities support core evidence sharing Clinical proposition & story as base for prioritizing HCP educational needs & gaps § Education integrated & built on latest SDD & RWE § Educational environment shaping based on key evidence Aligned communication to HCPs CLINICAL STORY & EVIDENCE A strong narrative links Med. Affair activities strategically and brings consistency in communication to HCP

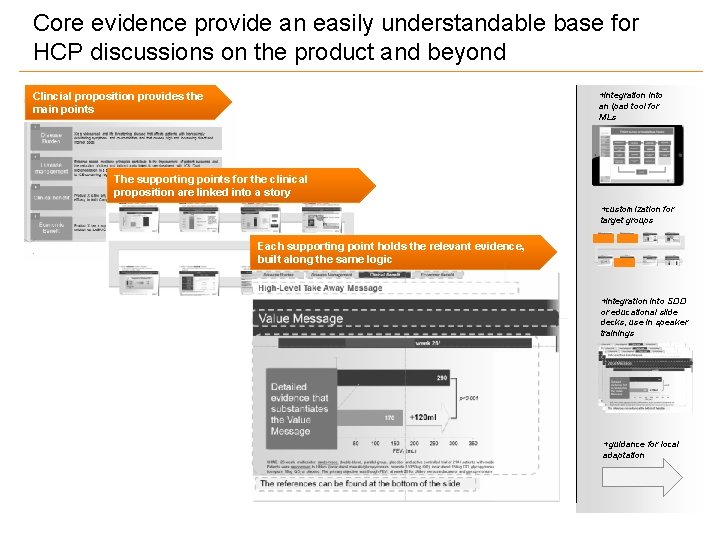
Core evidence provide an easily understandable base for HCP discussions on the product and beyond Clincial proposition provides the main points +integration into an ipad tool for MLs The supporting points for the clinical proposition are linked into a story +customization for target groups Each supporting point holds the relevant evidence, built along the same logic +integration into SDD or educational slide decks, use in speaker trainings +guidance for local adaptation

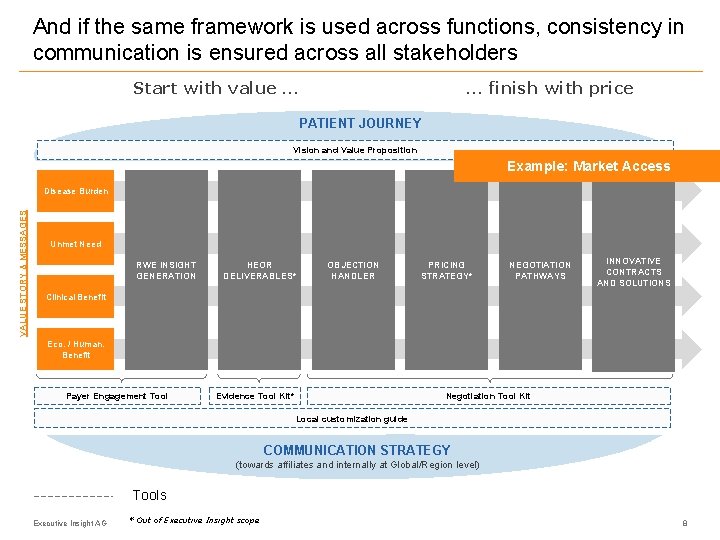
And if the same framework is used across functions, consistency in communication is ensured across all stakeholders Start with value … … finish with price PATIENT JOURNEY Vision and Value Proposition Example: Market Access VALUE STORY & MESSAGES Disease Burden Unmet Need RWE INSIGHT GENERATION HEOR DELIVERABLES* OBJECTION HANDLER PRICING STRATEGY* NEGOTIATION PATHWAYS INNOVATIVE CONTRACTS AND SOLUTIONS Clinical Benefit Eco. / Human. Benefit Payer Engagement Tool Evidence Tool Kit* Negotiation Tool Kit Local customization guide COMMUNICATION STRATEGY (towards affiliates and internally at Global/Region level) Tools Executive Insight AG * Out of Executive Insight scope 8

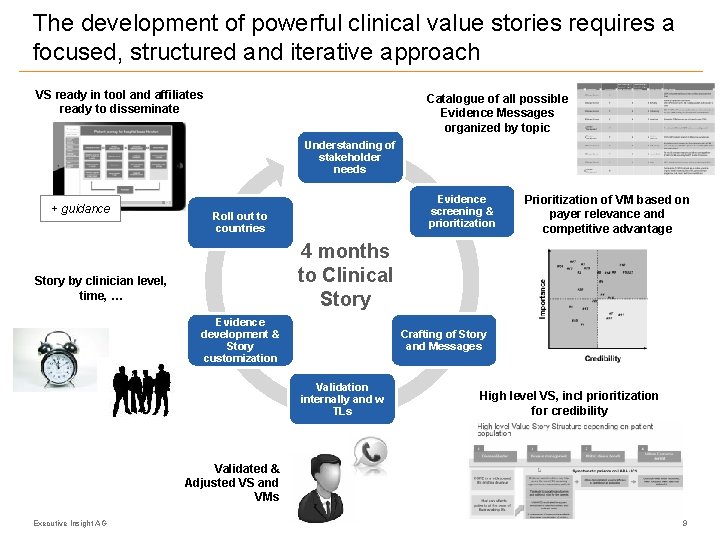
The development of powerful clinical value stories requires a focused, structured and iterative approach VS ready in tool and affiliates ready to disseminate Catalogue of all possible Evidence Messages organized by topic Understanding of stakeholder needs + guidance Evidence screening & prioritization Roll out to countries Prioritization of VM based on payer relevance and competitive advantage 4 months to Clinical Story by clinician level, time, … Evidence development & Story customization Crafting of Story and Messages Validation internally and w TLs High level VS, incl prioritization for credibility Validated & Adjusted VS and VMs Executive Insight AG 9

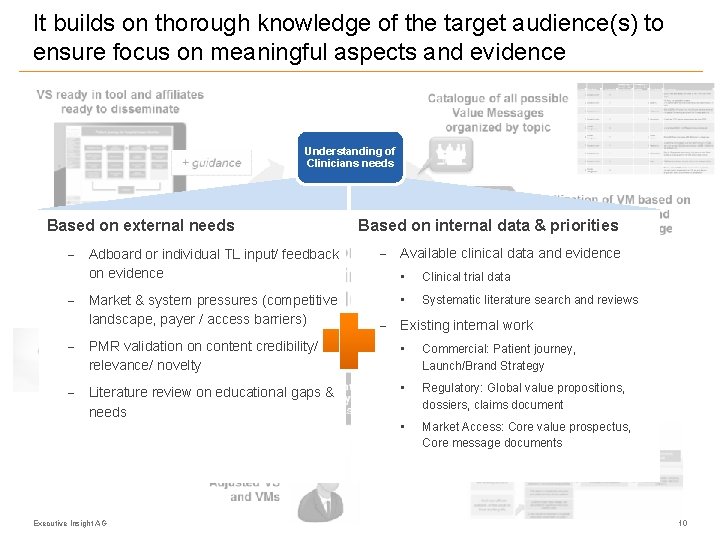
It builds on thorough knowledge of the target audience(s) to ensure focus on meaningful aspects and evidence Understanding of Clinicians needs Based on external needs - Adboard or individual TL input/ feedback on evidence - Market & system pressures (competitive landscape, payer / access barriers) Based on internal data & priorities - Available clinical data and evidence • Clinical trial data • Systematic literature search and reviews - Existing internal work - PMR validation on content credibility/ relevance/ novelty • Commercial: Patient journey, Launch/Brand Strategy - Literature review on educational gaps & needs • Regulatory: Global value propositions, dossiers, claims document • Market Access: Core value prospectus, Core message documents Executive Insight AG 10

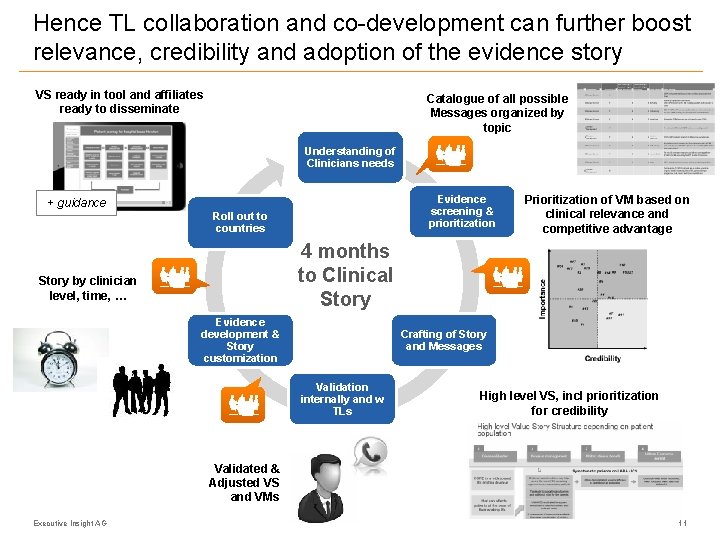
Hence TL collaboration and co-development can further boost relevance, credibility and adoption of the evidence story VS ready in tool and affiliates ready to disseminate Catalogue of all possible Messages organized by topic Understanding of Clinicians needs Evidence screening & prioritization + guidance Roll out to countries Prioritization of VM based on clinical relevance and competitive advantage 4 months to Clinical Story by clinician level, time, … Evidence development & Story customization Crafting of Story and Messages Validation internally and w TLs High level VS, incl prioritization for credibility Validated & Adjusted VS and VMs Executive Insight AG 11

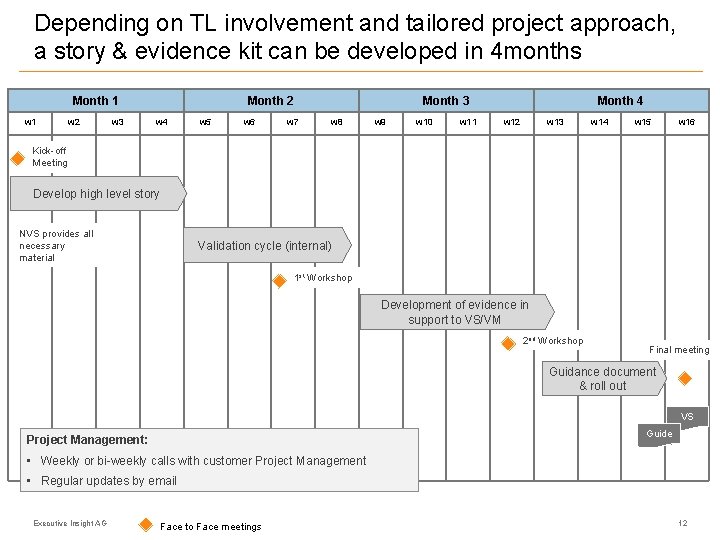
Depending on TL involvement and tailored project approach, a story & evidence kit can be developed in 4 months Month 1 w 2 w 3 Month 2 w 4 w 5 w 6 w 7 Month 3 w 8 w 9 w 10 w 11 Month 4 w 12 w 13 w 14 w 15 w 16 Kick-off Meeting Develop high level story NVS provides all necessary material Validation cycle (internal) 1 st Workshop Development of evidence in support to VS/VM 2 nd Workshop Final meeting Guidance document & roll out VS Guide Project Management: • Weekly or bi-weekly calls with customer Project Management • Regular updates by email Executive Insight AG Face to Face meetings 12
 Secondary sources
Secondary sources Primary evidence vs secondary evidence
Primary evidence vs secondary evidence Primary evidence vs secondary evidence
Primary evidence vs secondary evidence Primary evidence vs secondary evidence
Primary evidence vs secondary evidence Primary evidence vs secondary evidence
Primary evidence vs secondary evidence Why are fibers considered class evidence
Why are fibers considered class evidence Class vs individual evidence
Class vs individual evidence Explain how class evidence can have probative value
Explain how class evidence can have probative value A pair of latex gloves was found at a crime scene
A pair of latex gloves was found at a crime scene Define ecological fallacy
Define ecological fallacy Student engagement insight
Student engagement insight It is the controlling idea or central insight of the story.
It is the controlling idea or central insight of the story. Mse example
Mse example























