ERASE Trial The ERASE Trial Effect of r


- Slides: 9

ERASE Trial The ERASE Trial: Effect of r. HDL on Atherosclerosis-Safety and Efficacy Presented at American College of Cardiology Annual Scientific Session 2007 Presented by Dr. Jean-Claude Tardif, M. D. Clinical Trial Results. org

ERASE Trial: Background • CSL-111 is an apolipoprotein A-I isolated from human plasma and phosphatidylcholine derived from soybean. • Commercial development of the cholesteryl ester transfer protein inhibitor torcetrapib was recently stopped after a trial showed an increase in mortality with torcetrapib. Clinical Trial Results. org ACC 2007

ERASE Trial: Background • CSL-111 is a different class of agents targeting increasing HDL and is not a CETP inhibitor but is derived from human plasma. • The goal of this trial was to evaluate the effect of plaque burden of reconstituted HDL compared with placebo among patients with recent acute coronary syndromes. Clinical Trial Results. org ACC 2007

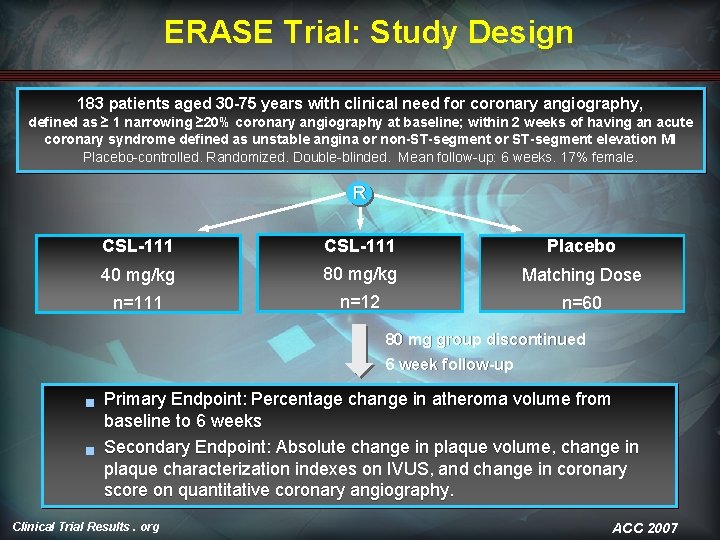
ERASE Trial: Study Design 183 patients aged 30 -75 years with clinical need for coronary angiography, defined as ≥ 1 narrowing ≥ 20% coronary angiography at baseline; within 2 weeks of having an acute coronary syndrome defined as unstable angina or non-ST-segment or ST-segment elevation MI Placebo-controlled. Randomized. Double-blinded. Mean follow-up: 6 weeks. 17% female. R CSL-111 Placebo 40 mg/kg 80 mg/kg Matching Dose n=111 n=12 n=60 80 mg group discontinued 6 week follow-up g g Primary Endpoint: Percentage change in atheroma volume from baseline to 6 weeks Secondary Endpoint: Absolute change in plaque volume, change in plaque characterization indexes on IVUS, and change in coronary score on quantitative coronary angiography. Clinical Trial Results. org ACC 2007

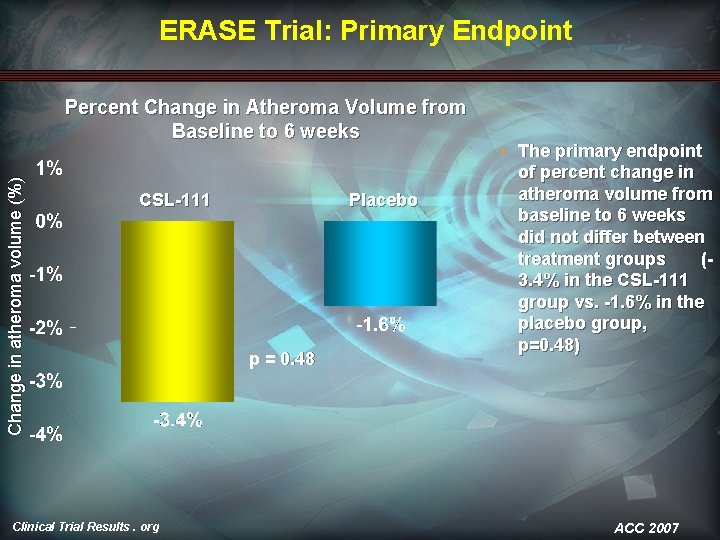
ERASE Trial: Primary Endpoint Change in atheroma volume (%) Percent Change in Atheroma Volume from Baseline to 6 weeks CSL-111 Clinical Trial Results. org Placebo p = 0. 48 • The primary endpoint of percent change in atheroma volume from baseline to 6 weeks did not differ between treatment groups (3. 4% in the CSL-111 group vs. -1. 6% in the placebo group, p=0. 48) ACC 2007

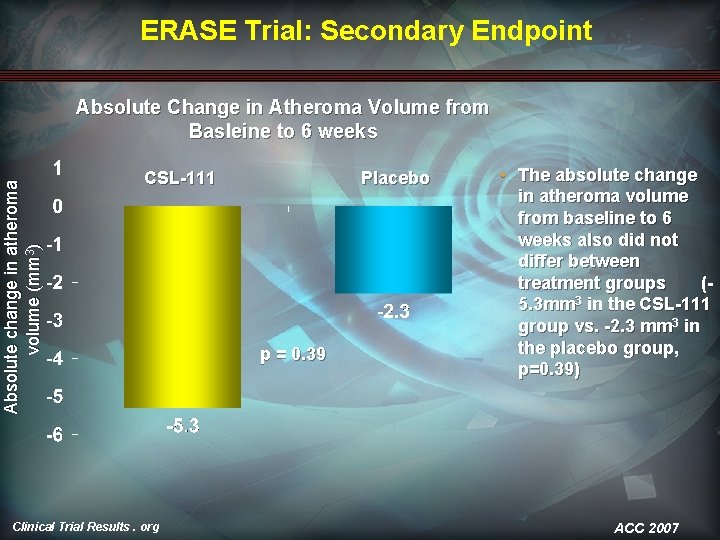
ERASE Trial: Secondary Endpoint Absolute change in atheroma volume (mm 3) Absolute Change in Atheroma Volume from Basleine to 6 weeks CSL-111 Clinical Trial Results. org Placebo p = 0. 39 • The absolute change in atheroma volume from baseline to 6 weeks also did not differ between treatment groups (5. 3 mm 3 in the CSL-111 group vs. -2. 3 mm 3 in the placebo group, p=0. 39) ACC 2007

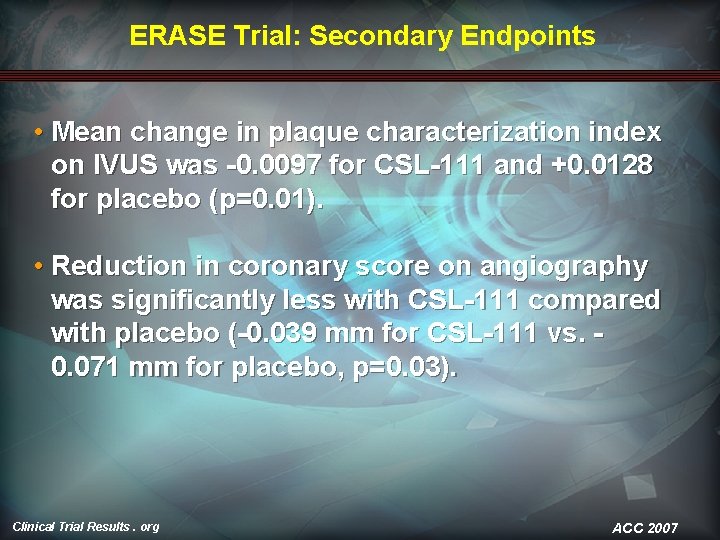
ERASE Trial: Secondary Endpoints • Mean change in plaque characterization index on IVUS was -0. 0097 for CSL-111 and +0. 0128 for placebo (p=0. 01). • Reduction in coronary score on angiography was significantly less with CSL-111 compared with placebo (-0. 039 mm for CSL-111 vs. 0. 071 mm for placebo, p=0. 03). Clinical Trial Results. org ACC 2007

ERASE Trial: Limitations • The 80 mg CSL-111 group was discontinued due to high rates of abnormal liver function tests, including alanine aminotransferase >5 times the upper limit of normal (ULN) in 50% of the group and aspartate aminotransferase >5 times ULN in 33% of the group. • The lipid effects of the agent in the study were not reported. • A larger trial would be required to evaluate the clinical safety and efficacy of this agent. Clinical Trial Results. org ACC 2007

ERASE Trial: Summary • Among patients with recent acute coronary syndromes, treatment with the novel reconstituted HDL agent CSL-111 was not associated with a reduction in atheroma volume compared with placebo. • Treatment with CSL-111 was associated with a change in atheroma volume within the treatment arm from baseline to 6 week follow-up. Clinical Trial Results. org ACC 2007