ELEMENTS Greek theory of physical world All earthly


- Slides: 7

ELEMENTS – Greek theory of physical world. All earthly objects are a mixture of: 1. EARTH (bottom – center of universe) 2. WATER (water covers earth) 3. AIR (air over water) 4. FIRE (highest – at top) 5. Ether = QUINTESSENCE (Latin) – substance whose natural motion is that most symmetrical and eternal of all conceivable motion = endless circles

ATOMIMC STRUCTURE • ATOMS – not ‘indivisible’ particles of Democritus and Dalton, but have electrons, protons, and neutrons. • Solar system model – central core (nucleus) with orbiting models • Scale • Atomic (Proton) Number = to the number of protons in nucleus. This number determines chemical species. • Ion – atom with net electrical charge due to loss/gain of one or more electrons • Isotopes – same atomic number, but different number of neutrons

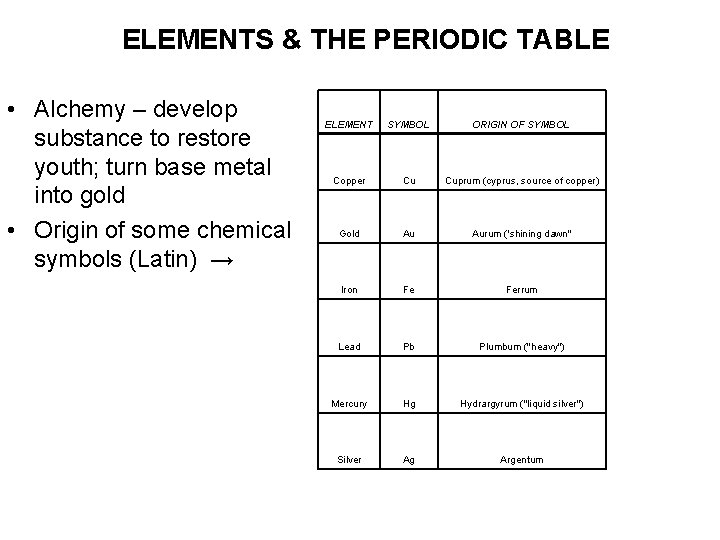
ELEMENTS & THE PERIODIC TABLE • Alchemy – develop substance to restore youth; turn base metal into gold • Origin of some chemical symbols (Latin) → ELEMENT SYMBOL ORIGIN OF SYMBOL Copper Cu Cuprum (cyprus, source of copper) Gold Au Aurum ('shining dawn" Iron Fe Ferrum Lead Pb Plumbum ("heavy") Mercury Hg Hydrargyrum ("liquid silver") Silver Ag Argentum

ELEMENTS – 92 natural elements MOLECULES – collection of atoms held by chemical bond ex. H 20 – water – 3 atoms C 6 H 12 O 6 – glucose – 24 atoms C 2 H 5 OH – alcohol – 9 atoms Chemical Bonding – involves electrons A. Ionic – transfer of electrons ex. Na. Cl (salt) : Na (ignites in air) Cl (poison gas) extra Na electron fills in outer Cl shell B. Covalent – mutual sharing of electrons ex. H 20 electrons most of the time of O leaving the two hydrogens positive.

States of Matter 1. Solid – definite shape, atoms locked into position, most have regular pattern. 2. Liquid – atoms packed tightly but not as tight as to prohibit movement; has surface. 3. Gas – large distances between atoms/molecules 4. Plasma – completely ionized gas ‘Atomic Theory’ – explains many things ex. Odors and evaporation

Aristotle (384 -322 B. C. ) • Greek philosopher, educator, scientist • Student of Plato

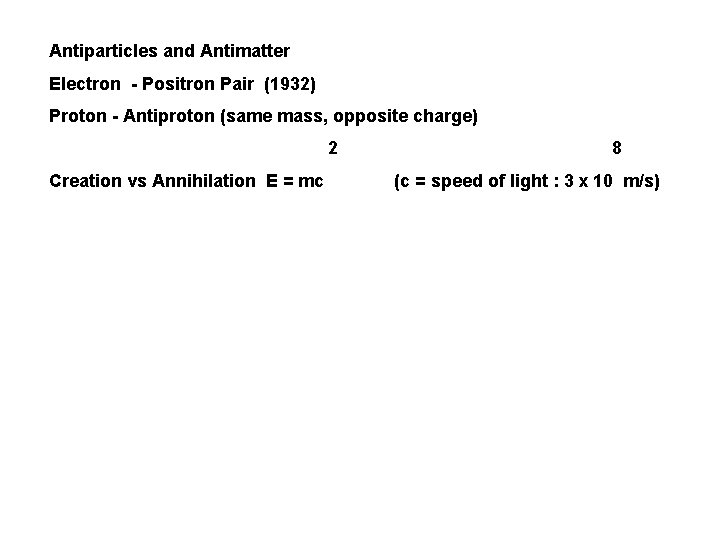
Antiparticles and Antimatter Electron - Positron Pair (1932) Proton - Antiproton (same mass, opposite charge) 2 Creation vs Annihilation E = mc 8 (c = speed of light : 3 x 10 m/s)













