EARTHS HISTORY RADIOMETRIC DATING TWO Ways to Date





- Slides: 10

EARTH’S HISTORY RADIOMETRIC DATING

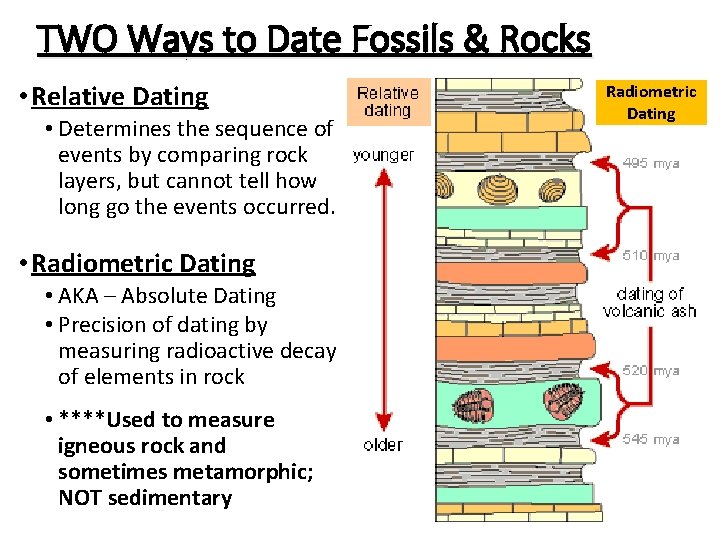
TWO Ways to Date Fossils & Rocks • Relative Dating • Determines the sequence of events by comparing rock layers, but cannot tell how long go the events occurred. • Radiometric Dating • AKA – Absolute Dating • Precision of dating by measuring radioactive decay of elements in rock • ****Used to measure igneous rock and sometimes metamorphic; NOT sedimentary Radiometric Dating

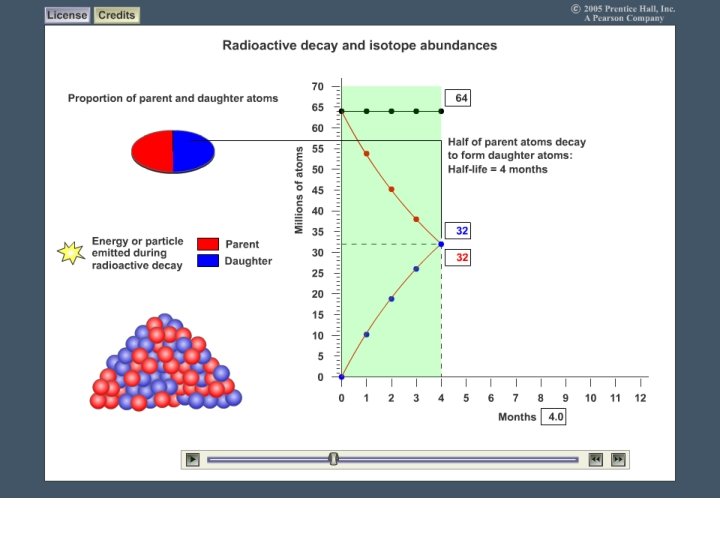
Radiometric Dating (Absolute Dating) • Radioactive Elements (or Isotopes): unstable atoms giving off atomic particles as radiation to become stable – this is called radioactive decay • Ex: Uranium-238 & Carbon-14 • Radiometric dating: Radioactive decay (going from unstable to stable) occurs at a constant rate called a half life. Each radioactive element has its own half life.

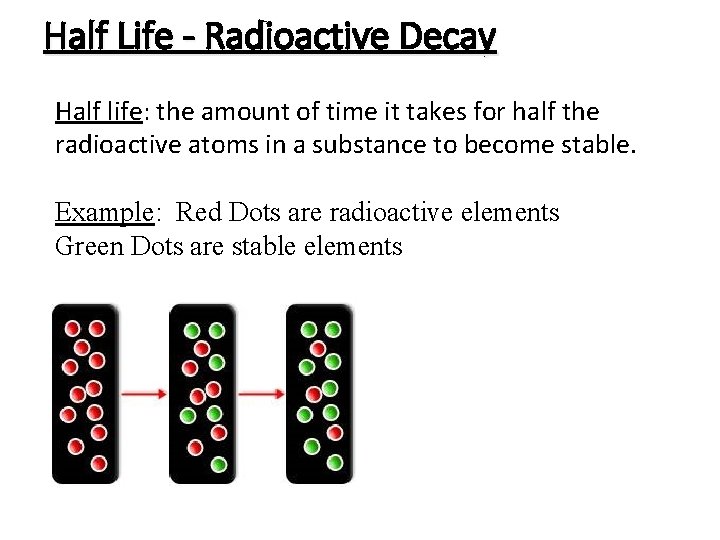
Half Life - Radioactive Decay Half life: the amount of time it takes for half the radioactive atoms in a substance to become stable. Example: Red Dots are radioactive elements Green Dots are stable elements

Ex: of Radioactive Elements (Isotopes) • Uranium-238 has a half-life of 4. 5 billion yrs (becomes Lead) • Potassium-40 has a half-life of 1. 3 billion yrs (becomes Argon-40) • Carbon-14 has a half-life of 5730 yrs (becomes Nitrogen) – AKA – Radiocarbon Dating – used to date once living material • Which would you use to date a wooly mammoth bone? • Which would you use to date igneous rock that may be the oldest sample on North America?

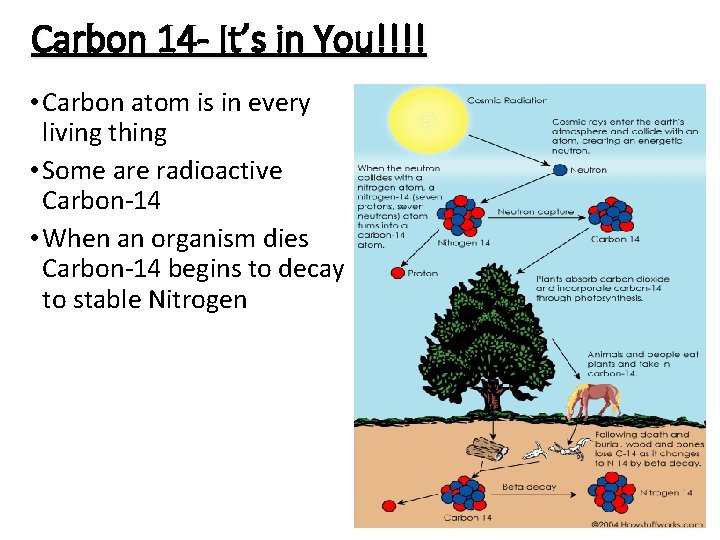
Carbon 14 - It’s in You!!!! • Carbon atom is in every living thing • Some are radioactive Carbon-14 • When an organism dies Carbon-14 begins to decay to stable Nitrogen

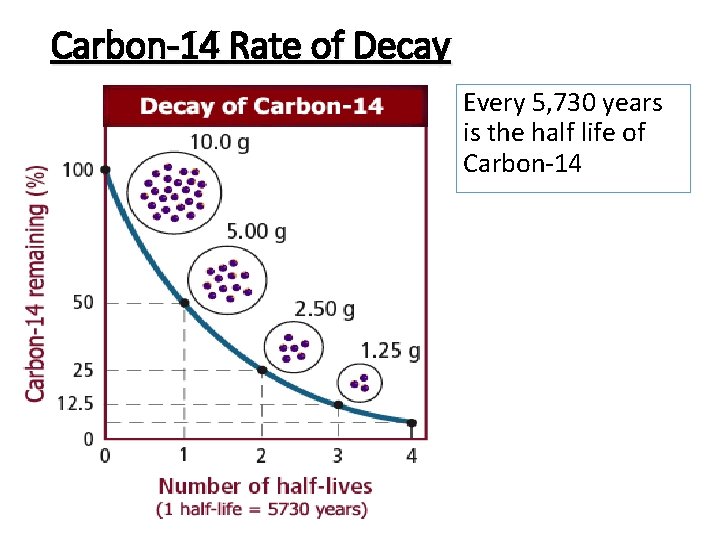
Carbon-14 Rate of Decay Every 5, 730 years is the half life of Carbon-14

Example of Radiocarbon Dating • Scientists were able to determine the age of the Iceman. • 5, 300 years old • Used the decay of radioactive carbon


Practice • What percentage of a radioactive element will be left after: 1 half-life ______ 2 half-lives _______ 3 half-lives ______ • A radioactive isotope has a half-life of 2, 000 years. Using a 10 gram sample. How much will remain radioactive after 2, 000 years, 4, 000 years and 6, 000 years? 2, 000 yrs _____ 4, 000 yrs ____ 6, 000 yrs _____