Dolutegravir versus Raltegravir in Treatment Experienced SAILING Study



- Slides: 6

Dolutegravir versus Raltegravir in Treatment Experienced SAILING Study

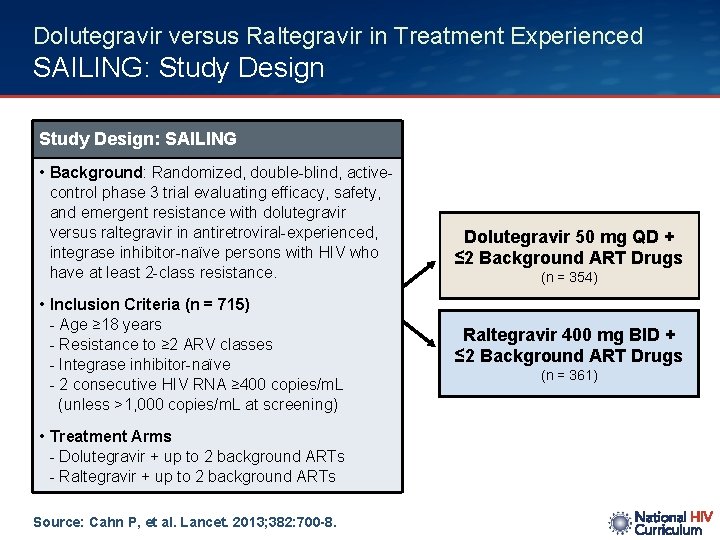
Dolutegravir versus Raltegravir in Treatment Experienced SAILING: Study Design: SAILING • Background: Randomized, double-blind, activecontrol phase 3 trial evaluating efficacy, safety, and emergent resistance with dolutegravir versus raltegravir in antiretroviral-experienced, integrase inhibitor-naïve persons with HIV who have at least 2 -class resistance. • Inclusion Criteria (n = 715) - Age ≥ 18 years - Resistance to ≥ 2 ARV classes - Integrase inhibitor-naïve - 2 consecutive HIV RNA ≥ 400 copies/m. L (unless >1, 000 copies/m. L at screening) • Treatment Arms - Dolutegravir + up to 2 background ARTs - Raltegravir + up to 2 background ARTs Source: Cahn P, et al. Lancet. 2013; 382: 700 -8. Dolutegravir 50 mg QD + ≤ 2 Background ART Drugs (n = 354) Raltegravir 400 mg BID + ≤ 2 Background ART Drugs (n = 361)

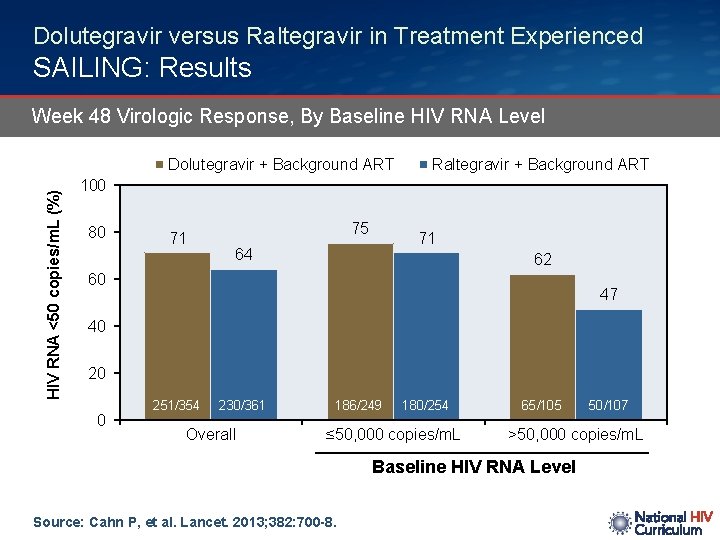
Dolutegravir versus Raltegravir in Treatment Experienced SAILING: Results Week 48 Virologic Response, By Baseline HIV RNA Level HIV RNA <50 copies/m. L (%) Dolutegravir + Background ART Raltegravir + Background ART 100 80 71 75 71 64 62 60 47 40 20 0 251/354 230/361 Overall 186/249 180/254 ≤ 50, 000 copies/m. L 65/105 >50, 000 copies/m. L Baseline HIV RNA Level Source: Cahn P, et al. Lancet. 2013; 382: 700 -8. 50/107

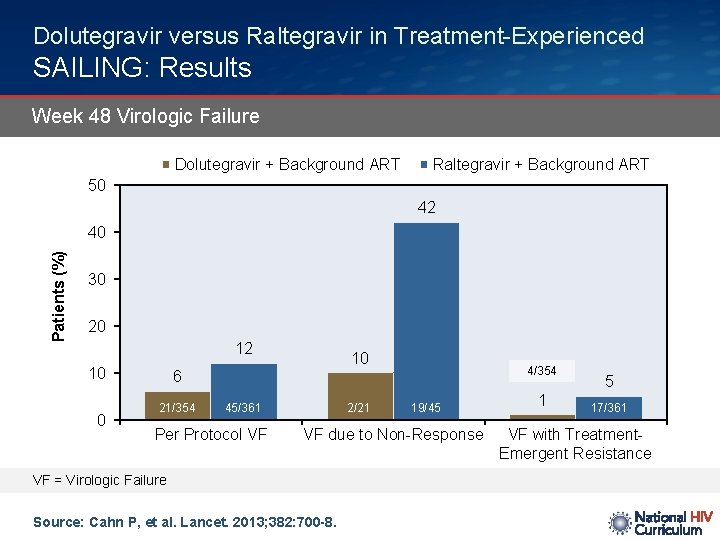
Dolutegravir versus Raltegravir in Treatment-Experienced SAILING: Results Week 48 Virologic Failure Dolutegravir + Background ART Raltegravir + Background ART 50 42 Patients (%) 40 30 20 12 10 0 10 4/354 6 21/354 45/361 Per Protocol VF 2/21 19/45 VF due to Non-Response VF = Virologic Failure Source: Cahn P, et al. Lancet. 2013; 382: 700 -8. 1 5 17/361 VF with Treatment. Emergent Resistance

Dolutegravir versus Raltegravir in Treatment Experienced SAILING Study: Conclusion Interpretation: “Once-daily dolutegravir, in combination with up to two other antiretroviral drugs, is well tolerated with greater virological effect compared with twice-daily raltegravir in this treatment-experienced patient group. ” Source: Cahn P, et al. Lancet. 2013; 382: 700 -8.

Acknowledgment The National HIV Curriculum is an AIDS Education and Training Center (AETC) Program supported by the Health Resources and Services Administration (HRSA) of the U. S. Department of Health and Human Services (HHS) as part of an award totaling $800, 000 with 0% financed with non-governmental sources. This project is led by the University of Washington’s Infectious Diseases Education and Assessment (IDEA) Program. The content in this presentation are those of the author(s) and do not necessarily represent the official views of, nor an endorsement, by HRSA, HHS, or the U. S. Government.











