Do Now In your own words define a














- Slides: 23

Do Now • In your own words, define a gas • In your own words, define pressure • What is the molarity if 10 g of KCl in 2 L of solution?

Aim • What are the variables in gases?

Agenda • • Do Now Mini-Lesson Ph. ET Simulation Lab Castle learning Regents Review

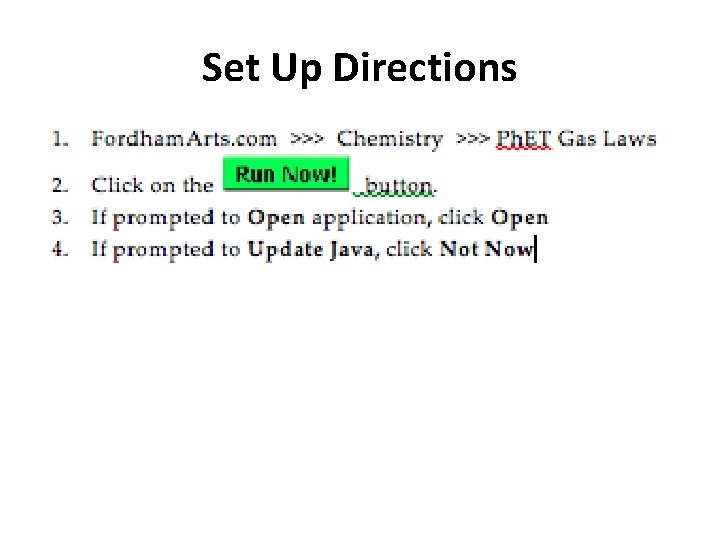
Set Up Directions

Exploration 1: Temperature and Pressure • Pump in some light species gas into the container • Pick “Volume” as the constant parameter since you do not want it to change. • Vary the temperature, and then wait until pressure stops fluctuating to a very high degree. Write in the data. • Copy all data tables and graphs onto Loose Leaf paper


• Change the volume of the container by taking the constant parameter off. Move the box to a different location. • Use the ruler to find the new size of the box. Write this measurement here: __________ • Repeat the experiment in step 2 using the same temperature values.


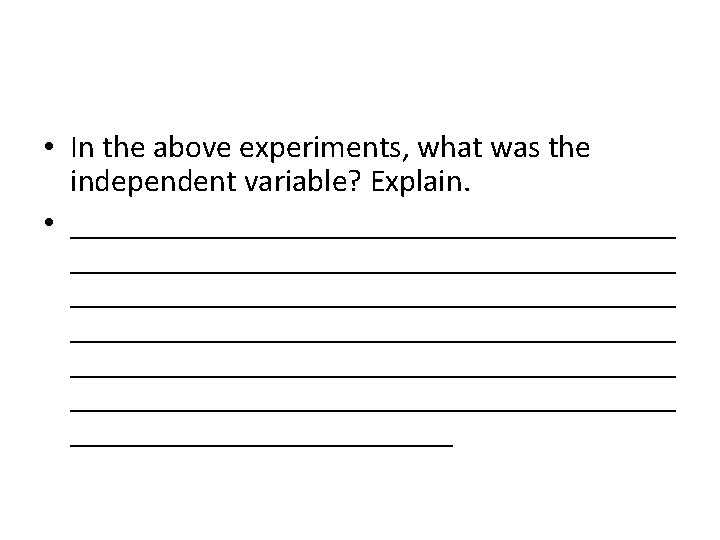
• In the above experiments, what was the independent variable? Explain. • ______________________________________ ______________________________________

• In the above experiments, what was the dependent variable? Explain. • ______________________________________ ______________________________________

• Create a scatter plot for Temperature vs. Pressure for BOTH situations. Include a legend to differentiate the data points. – Label your axes with the independent and dependent variables – SCALE both axes. – Plot the data sets using DIFFERENT symbols

Legend

• Develop a scientific explanation for the relationship that is present in the above graph. In doing so, consider the following questions: – Why did the amount of pressure change with different amounts of temperature? – Did the slope of the line change for different volumes? Why or why not?

• Exploration 2: Volume and Pressure • Pump in some light species gas into the container • Pick an initial temperature then pick “Temperature” as the constant parameter. – Write this temperature here: __________ • Vary the volume, and then wait until the temperature settles back to the ORIGINAL value. Write the data below. • Use the ruler to measure the volume. Move the side of the box to change the volume.

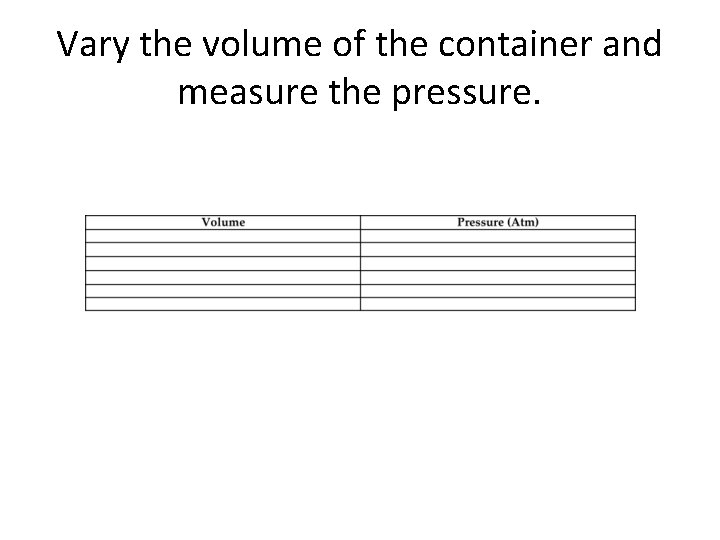
Vary the volume of the container and measure the pressure.

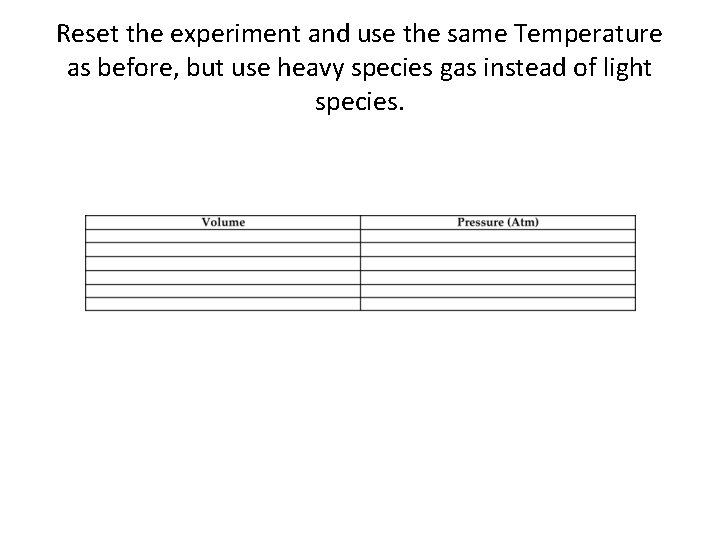
Reset the experiment and use the same Temperature as before, but use heavy species gas instead of light species.

• In the above experiments, what was the independent variable? Explain. ______________________________________ ______________________________________

• In the above experiments, what was the dependent variable? Explain. • ________________________________________ ________________________________________

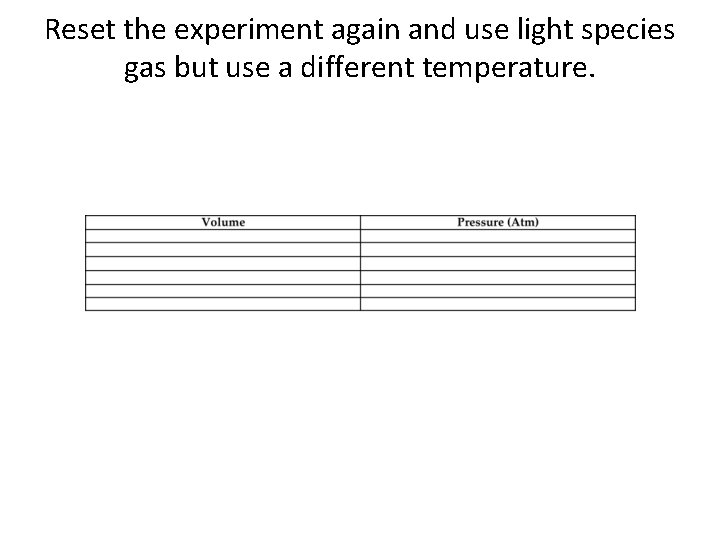
Reset the experiment again and use light species gas but use a different temperature.

• Create a scatter plot for Volume vs. Pressure for BOTH species of gas. Include a legend to differentiate the data points. – Label your axes with the independent and dependent variables – SCALE both axes. – Plot the data sets using DIFFERENT symbols

Legend

• Develop a scientific explanation for the relationship that is present in the above graph. In doing so, consider the following questions: – Why did the amount of pressure change with different amounts of volume? Explain using logic, chemistry knowledge, and common sense. – Did the shape of the line change for different species of gas? How do these results compare to your results from experiment #1?

• What two conclusions can you make about relationships between variables given the two experiments you conducted? Support with data and trends from graphs.