Crucial Postapproval Caveats for Any Percutaneous Valve Trial








- Slides: 10

Crucial Post-approval Caveats for Any Percutaneous Valve Trial by Daniel Caños, MPH Team Leader, Cardiovascular Devices Division of Epidemiology Office of Surveillance and Biometrics Food and Drug Administration

Post-Approval Studies (PAS) • Which are sometimes required studies on class III, PMA devices • Ordered at time of approval • Authority under CFR Title 21 Section 814. 82 (a) FDA may impose post-approval requirements at the time of approval of the PMA … (2) Continuing evaluation and reporting on the safety, effectiveness, and reliability of the device for its intended use. . . Monday 2/22 3: 05 PM

Need for Post-Approval Studies • Gather essential postmarket information – Long-term performance including effects of retreatments & device changes – Real-world device performance (patients and clinicians) – Effectiveness of training programs – Sub-group performance – Outcomes of concern (safety and effectiveness) Monday 2/22 3: 05 PM

PAS Public Health Value • Evaluate medical devices as they enter a “ realworld” utilization • Contribute to better design of premarket studies • Can provide infrastructure for nesting premarket clinical trials • Can detect real-time signals (actionable) • Help identify over-arching regulatory science needs • Help prioritize CDRH epidemiologic research resources Monday 2/22 3: 05 PM

General Principles for Post. Approval Studies • Objective is to evaluate device performance and potential device-related problems in a broader population over an extended period of time after premarket establishment of reasonable evidence of device safety and effectiveness. • Post-approval studies should not be used to evaluate unresolved issues from the premarket phase that are important to the initial establishment of device safety and effectiveness. Monday 2/22 3: 05 PM

PAS Development Considerations • Consent IDE patients for long-term followup • Early consideration of post-market questions • Interactive protocol development with the FDA • Implement methods for maximizing participant retention Monday 2/22 3: 05 PM

Post-Approval Study Components • • • Fundamental study question or hypothesis Study design and study groups (including description of control group) Safety and effectiveness endpoints and methods of assessment Projected sample size Statistical analysis plan Monday 2/22 3: 05 PM

Post-Approval Study Components • Duration of follow-up • Follow-up schedule and plan to minimize loss to follow-up • Study timeline Monday 2/22 3: 05 PM

Monday 2/22 3: 05 PM

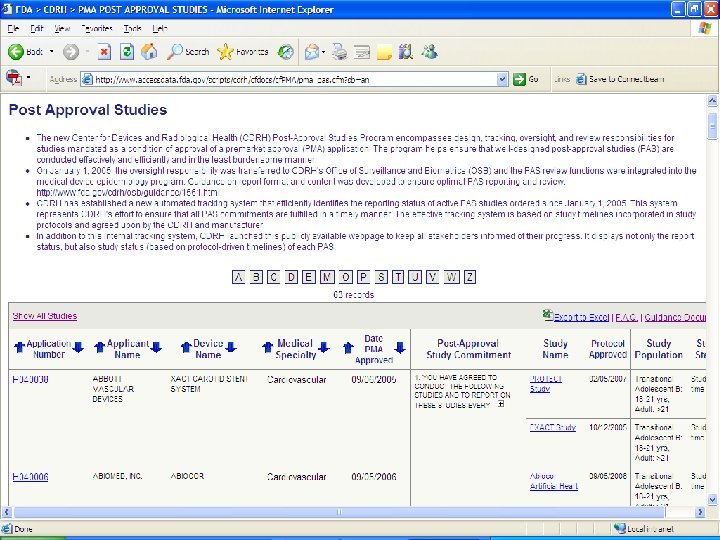
For More Information Email: daniel. canos@fda. hhs. gov Post-approval study status website: http: //www. accessdata. fda. gov/scripts/cdrh/c fdocs/cfpma/pma_pas. cfm Procedures for Handling Post-Approval Studies Imposed by PMA Order: http: //www. fda. gov/Medical. Devices/Device. R egulationand. Guidance/Guidance. Documents /ucm 070974. htm Monday 2/22 3: 05 PM