Competition Assessment of the Indian Pharmaceuticals Sector Aditya










- Slides: 12

Competition Assessment of the Indian Pharmaceuticals Sector Aditya Bhattacharjea Fiyanshu Sindhwani Centre for Development Economics, Delhi School of Economics

Structure of the report �Introduction ◦ Importance of drug availability and pricing in India ◦ Special features of the market for medicines �Evolution of the policy regime �Empirical analysis of market structure �Competition law �Drug price control �Foreign direct investment—takeovers �TRIPS and patent protection �Public production, procurement and distribution �Competition assessment checklist

Evolution of policy regime � 1970: Patents Act and DPCO � 1973 FERA �Restrictive trade policy �MRTP Act �Progressive from 1990 s relaxation of all the above

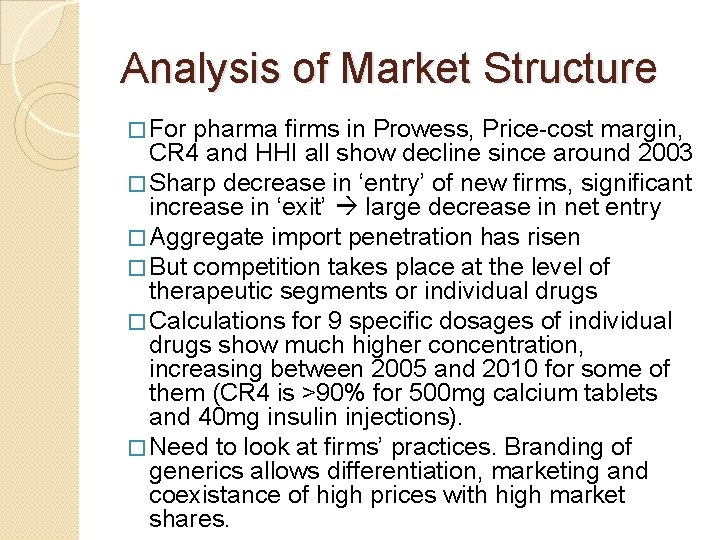
Analysis of Market Structure � For pharma firms in Prowess, Price-cost margin, CR 4 and HHI all show decline since around 2003 � Sharp decrease in ‘entry’ of new firms, significant increase in ‘exit’ large decrease in net entry � Aggregate import penetration has risen � But competition takes place at the level of therapeutic segments or individual drugs � Calculations for 9 specific dosages of individual drugs show much higher concentration, increasing between 2005 and 2010 for some of them (CR 4 is >90% for 500 mg calcium tablets and 40 mg insulin injections). � Need to look at firms’ practices. Branding of generics allows differentiation, marketing and coexistance of high prices with high market shares.

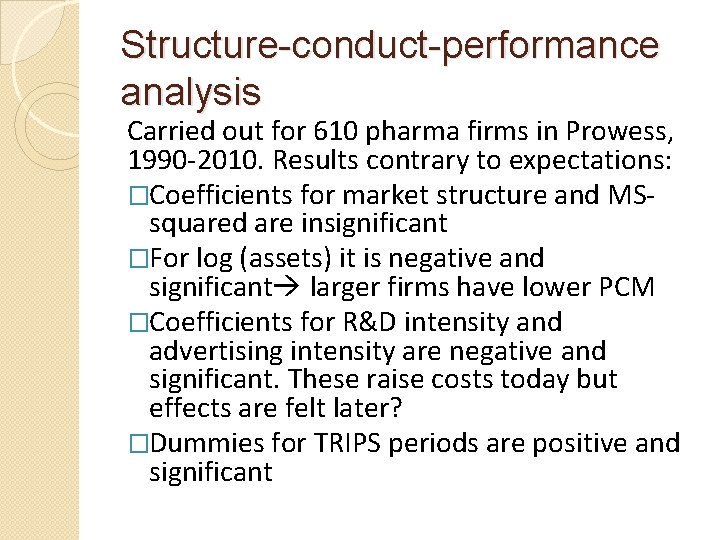
Structure-conduct-performance analysis Carried out for 610 pharma firms in Prowess, 1990 -2010. Results contrary to expectations: �Coefficients for market structure and MSsquared are insignificant �For log (assets) it is negative and significant larger firms have lower PCM �Coefficients for R&D intensity and advertising intensity are negative and significant. These raise costs today but effects are felt later? �Dummies for TRIPS periods are positive and significant

Competition law �Ineffectiveness of MRTP Act �Review of all pharma-related cases decided till now under Competition Act ◦ Two cases of regional chemists’ & druggists’ associations forcing manufacturers to limit number of stockists, restrict bidders for government procurement, and fix trade margins �These practices are apparently carried out nationwide – need for wider inquiry �Fine based on association’s turnover grossly inadequate �Fixation of trade margins by NPPA: RPM by govt mandate?

Merger cases � 6 pharma cases decided since June 2011; all were approved. ü The ultimate control over the parties in the combination remains the same before and after the combination (intra-group reorganization). ü Companies not engaged in similar businesses and no vertical integration (conglomerate merger). ü Absence of one of the parties in India in the business of the other party ü Significant presence of other players (no AAEC) CCI modified non-compete agreement in one instance � But we have identified several mergers that were not screened because the combined assets or turnovers of the firms were below the thresholds specified in the Act, or the assets/turnover of the target was below the threshold specified by the 2011 notification. Case for reviewing thresholds for this sector? �

Drug Price Control �Review of debate over transition from cost-based pricing of 74 drugs under DPCO 1995 to market-based formula for all 348 NLEM drugs in NPPP-2012. ◦ Weaknesses in arguments on both sides ◦ Price controls are usually imposed for natural monopolies where the number of products and producers is few and competition infeasible. ◦ Possibility that controlled prices can be used as focal prices for facilitating oligopolistic coordination – parallel with cement case?

Foreign Direct Investment and Takeovers � Critical review of Maira Committee Report � Review of debate on role of MNCs ◦ Screening by CCI (with extra expertise on health issues) preferable to FIPB. ◦ Case for reducing merger review thresholds – Competition (Amendment) Bill 2012. ◦ Market share of foreign firms has not gone up post. TRIPS ◦ But they are increasingly supplying the market through imports, esp of high priced patented drugs and also generics ◦ Effects of takeover on R&D inconclusive, but foreign firms overall have much lower R&D intensity ◦ Too early to detect impact of 2008 -10 foreign takeovers?

IPR Issues – Impact of TRIPS �Some evidence that growth rate of R&D expenditure and the number of process and product patents filed by leading Indian firms declined after 2005. �R&D for drugs to treat diseases of greatest public health importance (malaria, TB) neglected in favour of ‘lifestyle’ diseases by both Indian and foreign firms. �Encouraging signs of India using TRIPS flexibilities: �Grant of compulsory licenses for Bayer’s Nexavar and now 3 more cancer drugs �Use of 3(d) to deny evergreening patent for minor improvements in Novartis’s Glivec

Public production and procurement � In 2008, Health Ministry closed down 3 leading PSUs on grounds of not complying with GMPs. Reopened in 2010 but production still far below earlier levels govt has to procure vaccines from private producers at much higher prices. � Government procurement rules to ensure GMPs have been struck down by High Courts as excluding competition without adequate justification. � Need to provide assistance to smaller units to comply with GMP and to enforce quality standards under Drugs and Cosmetics Act. This would increase competition for bidding and also weaken industry’s argument against debranding.

THANK YOU! �Comments welcome: aditya@econdse. org
 Monopoly characteristics
Monopoly characteristics Monopoly vs monopolistic competition
Monopoly vs monopolistic competition Market structures venn diagram
Market structures venn diagram Competition refers to
Competition refers to Introduction to icici bank
Introduction to icici bank Triumph pharmaceuticals
Triumph pharmaceuticals What is contoso
What is contoso Contoso pharmaceuticals
Contoso pharmaceuticals Contoso pharmaceuticals
Contoso pharmaceuticals Contoso presentation
Contoso presentation Contoso pharmaceuticals
Contoso pharmaceuticals Contoso pharmaceuticals
Contoso pharmaceuticals Ambalal sarabhai products list
Ambalal sarabhai products list