CLEAN HANDS A simple act towards preventing deadly





















- Slides: 29

CLEAN HANDS: A simple act towards preventing deadly device associated infection IVDAIs Hand Hygiene Day, Egypt 2015 Walaa Abdelatif

• Prospective studies in which every IVD was cultured at the timeof removal show that every type of device carries some risk ofcausing BSI, but that the magnitude of risk varies greatly. • The device that poses the greatest risk of IVDR BSI today isthe central venous catheter (CVC) in its many forms: up to 75% of IVDR BSIs originate from CVCs of various types • and CVCs are the most important risk factor for nosocomial candidemia

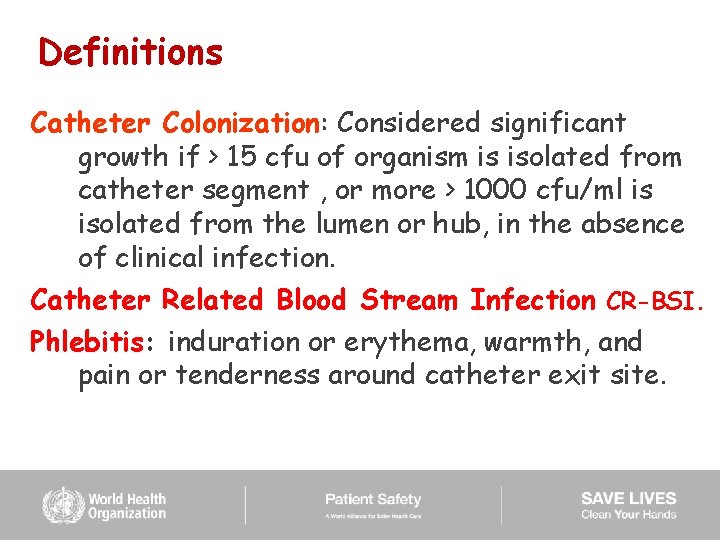
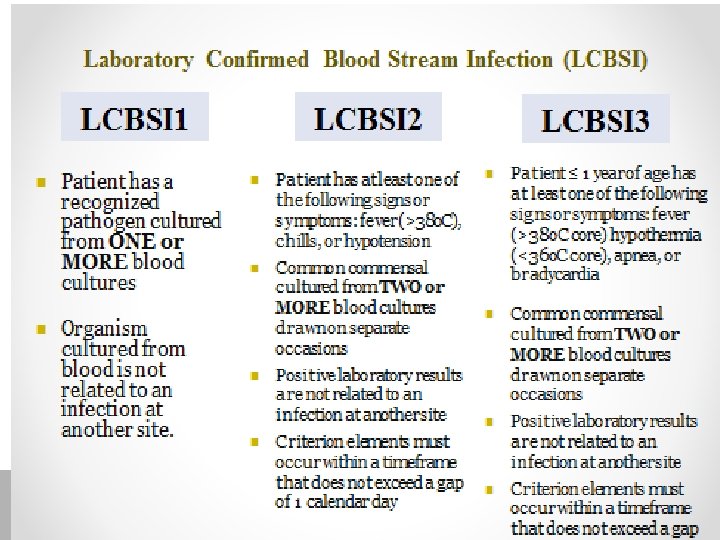
Definitions Catheter Colonization: Considered significant growth if > 15 cfu of organism is isolated from catheter segment , or more > 1000 cfu/ml is isolated from the lumen or hub, in the absence of clinical infection. Catheter Related Blood Stream Infection CR-BSI. Phlebitis: induration or erythema, warmth, and pain or tenderness around catheter exit site.




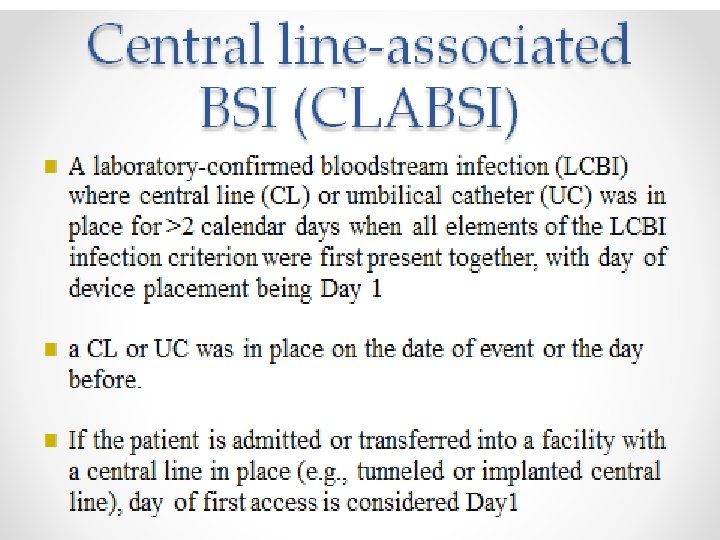
There are 2 major sources of IVDR BSI: colonization of the IVD contamination of the fluid administered

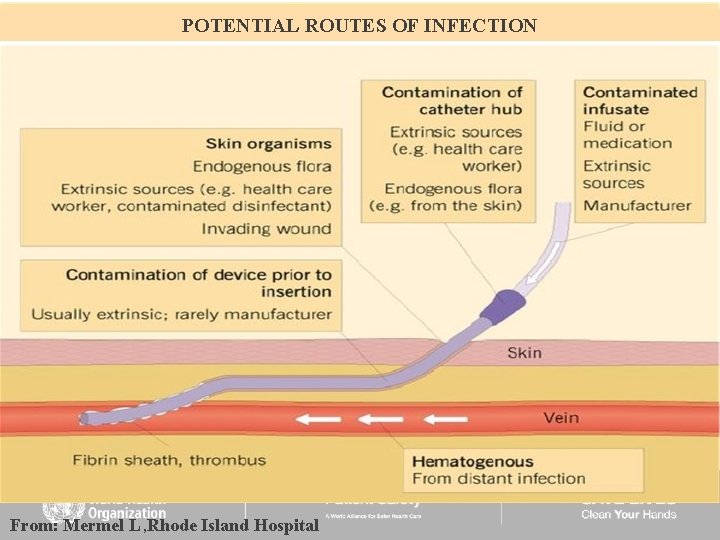
POTENTIAL ROUTES OF INFECTION From: Mermel L , Rhode Island Hospital

Biofilm Most infected urinary catheters are covered by a thick biofilm containing the infecting microorganisms embedded in a matrix of host proteins and microbial exoglycocalyx. A biofilm forms intraluminally, extraluminally, or both ways

catheter-related infection is caused by: Colonization of the external surfaces of by microorganisms from the patient’s skin around the insertion site. This can occur through contamination of the catheter tip at the time of insertion or migration of skin organisms at the insertion site into the cutaneous catheter tract after insertion.

Contamination of the catheter hub with distal spread of the organisms down the intraluminal surface. This is largely thought to occur during handling of the connections at catheter junctions

3. Occasionally, the catheter might become haematogenously seeded from another focus of infection. 4. Rarely, by contamination of the fluid infusate

• Sepsis appearing within a short time period after catheter insertion is usually due to skin contamination, • while hub contamination results in bloodstream infection occurring after the first week of line duration

In 2009, the Centers for Disease Control and Prevention (CDC) and Healthcare Infection Control Practices Advisory Committee (HICPAC) integrated current advances in guideline production and implementation into its development process (http: //www. cdc. gov/hicpac/guideline. Method. html).

These guidelines are intended to provide evidence-based recommendations for preventing intravascular catheterrelated infections.

The system for categorizing recommendations in this guideline is as follows: Category IA. Strongly recommended for implementation and strongly supported by well-designed experimental, clinical, or epidemiologic studies. Category IB. Strongly recommended for implementation and supported by some experimental, clinical, or epidemiologic studies and a strong theoretical rationale; or an accepted practice (e. g. , aseptic technique) supported by limited evidence. Category IC. Required by state or federal regulations, rules, or standards. Category II. Suggested for implementation and supported by suggestive clinical or epidemiologic studies or a theoretical rationale.

Unresolved issue. Represents an unresolved issue for which evidence is insufficient or no consensus regarding efficacy exists.

Summary of Recommendations 1) educating and training healthcare personnel who insert and maintain catheters 2) using maximal sterile barrier precautions during central venous catheter insertion 3) using a > 0. 5% chlorhexidine skin preparation with alcohol for antisepsis 4) avoiding routine replacement of central venous catheters as a strategy to prevent infection 5) using antiseptic/antibiotic impregnated short-term central venous catheters and chlorhexidine impregnated sponge dressings if the rate of infection is not decreasing

Antimicrobial/Antiseptic Impregnated Catheters and Cuffs • Use a chlorhexidine/silver sulfadiazine or minocycline/rifampin -impregnated CVC in patients whose catheter is expected to remain in place >5 days if, after successful implementation of a comprehensive strategy to reduce rates of CLABSI, the CLABSI rate is not decreasing. • The comprehensive strategy should include at least the following three components: 1. educating persons who insert and maintain catheters 2. use of maximal sterile barrier precautions 3. and a >0. 5% chlorhexidine preparation with alcohol for skin antisepsis during CVC insertion. (Category IA)

Do NOT think of treating local infection with local antibiotics ointment

Treating the garbage!!!!!

These guidelines also emphasize performance improvement by implementing bundled strategies, and documenting and all components of the bundle as benchmarks for quality assurance and performance improvement. reporting rates of compliance with

The Central Line Bundle …is a group of interventions related to patients with intravascular central catheters that, when implemented together, result in better outcomes than when implemented individually. *Bundle: Grouping of best practices

Recommendations to prevent CVCRIs(Central line bundle): 1. Hand Hygiene 2. Maximal barrier precautions (as surgical technique) 3. Chlorhexidine skin antisepsis 4. Optimal catheter site selection (subclavian vein is the preferred site for non-tunneled catheters) 5. Daily review of line necessity, with immediate removal of unnecessary lines


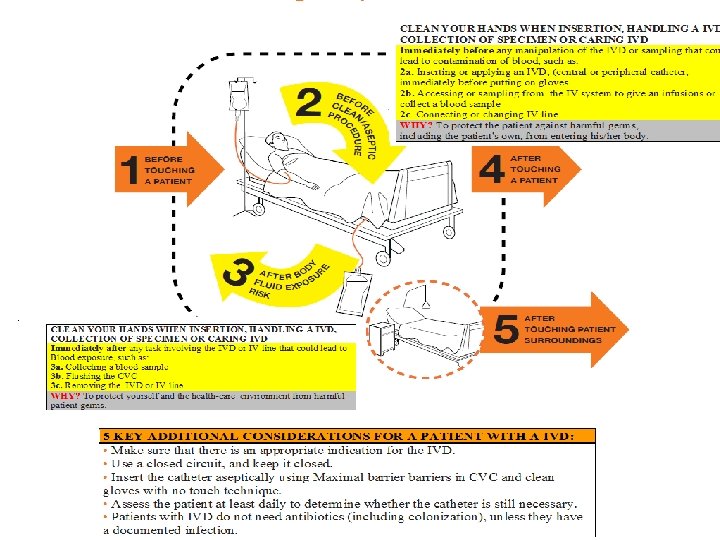
5 KEY ADDITIONAL CONSIDERATIONS FOR A PATIENT WITH A IVD: • Make sure that there is an appropriate indication for the IVD. • Use a closed circuit, and keep it closed. • Insert the catheter aseptically using Maximal barriers in CVC and clean gloves with no touch technique in PVC. • Assess the patient at least daily to determine whether the catheter is still necessary. • Patients with IVD do not need antibiotics (including colonization), unless they have a documented infection.

IVD. docx

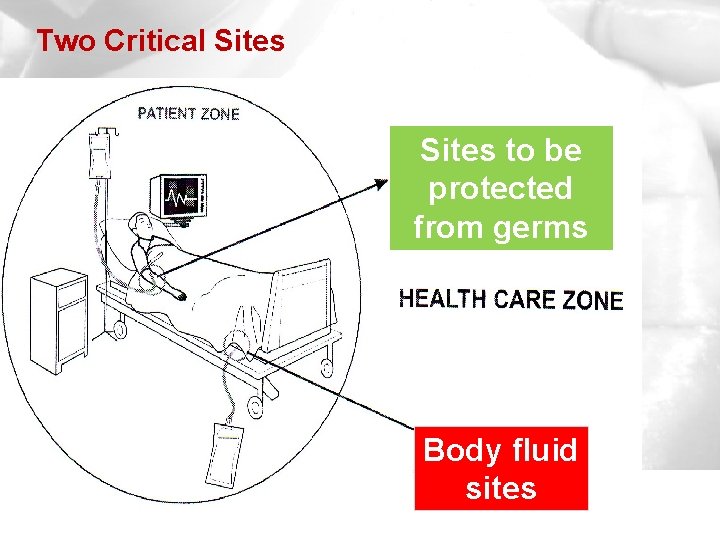
Two Critical Sites to be protected from germs Body fluid sites

Thank you