Chem 231 218 Lecture Announcements Set 2 Homework




- Slides: 18

Chem. 231 – 2/18 Lecture

Announcements • Set 2 Homework – Due Wednesday • Quiz 2 – Next Monday • Set 1 Labs – should be switching instruments today (or after today) – HPLC – instructions on setting up sequence for more efficient use of time • Extraction Labs – SPE/HPLC lab completed (hand out) – I hope to have SPME/GC lab ready soon

HPLC – Using Sequences • Demo showed how to analyze samples, one sample at a time • Using autosampler to run a sequence is useful to: – run multiple samples in a defined way (e. g. calibration standards after method optimization is done) – run samples while doing other work (preparing GC lab samples or going home) – if left running unattended, must use working “Exit” program

HPLC – Using Sequences • Sequences will be kept in Sequence Folder

HPLC – Using Sequences • Start a new sequence

HPLC – Using Sequences • Sequence Parameters and Table Need Setting

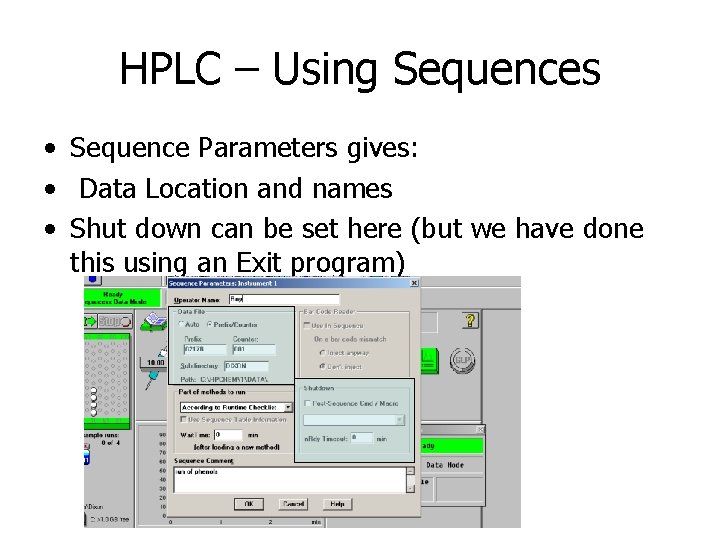
HPLC – Using Sequences • Sequence Parameters gives: • Data Location and names • Shut down can be set here (but we have done this using an Exit program)

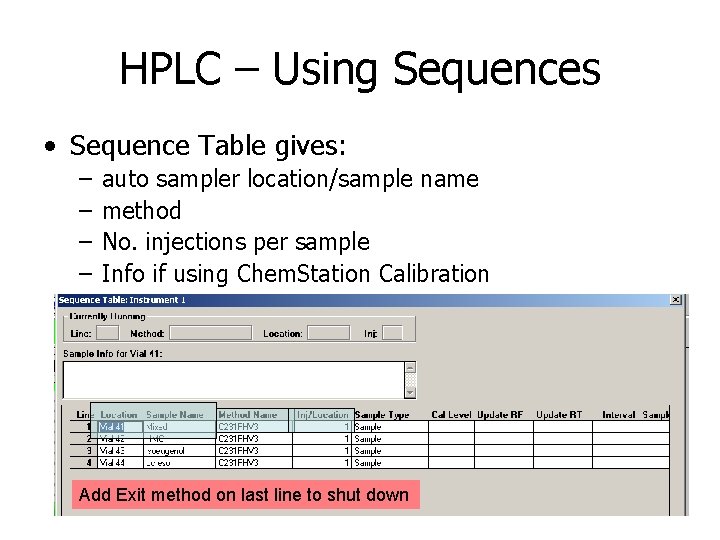
HPLC – Using Sequences • Sequence Table gives: – – auto sampler location/sample name method No. injections per sample Info if using Chem. Station Calibration Add Exit method on last line to shut down

Questions I On extractions, low performance LC, and integration 1. 2. 3. Fatty acids are being trapped using a C 18 SPE column and then analyzed by HPLC with a C 18 column (both under acidic conditions). The elution order observed on the column is: C 12, C 16: 1, C 14, C 18: 2, C 16, C 18: 1, C 18. Efficiency tests with C 14 show 98% recovery. Will all other fatty acids be retained with similar recovery? If not, which will have a risk of reduced recover? Furans are recovered on a diol type SPE cartridge out of transformer oil diluted in hexane. They are eluted using 50% methanol, 50% water. They are then analyzed using reversed phase HPLC. How can you use the HPLC elution order to predict poor trapping or elution efficiency on the SPE cartridge. If in the SPE analysis in question 2, the chemist forgot to wash the SPE cartridge with hexane after sample application, what would be the consequence?

Questions II On extractions, low performance LC, and integration 1. 2. Under which of the following conditions would you want to use low performance LC? a) separation of chemically similar natural products from a plant sample to obtain mg quantities b) separation of mg quantities of reaction products from chemically dissimilar reactants and side products c) analysis of ng quantities of pesticides from food samples d) separation of mg quantities of reaction products from chemically dissimilar reactants and side products When is it important to set the integration criteria carefully to distinguish between noise and peaks? a) when peaks have small signal to noise ratios b) when many peaks overlap c) when peak width changes over the chromatogram

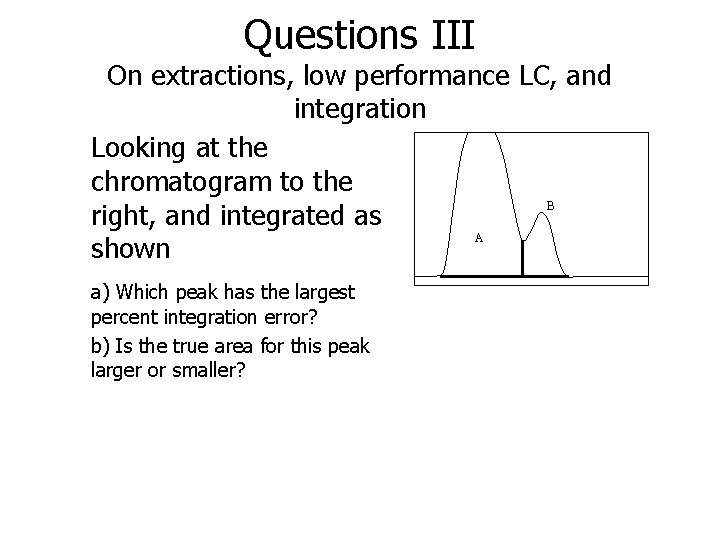
Questions III On extractions, low performance LC, and integration Looking at the chromatogram to the B right, and integrated as A shown a) Which peak has the largest percent integration error? b) Is the true area for this peak larger or smaller?

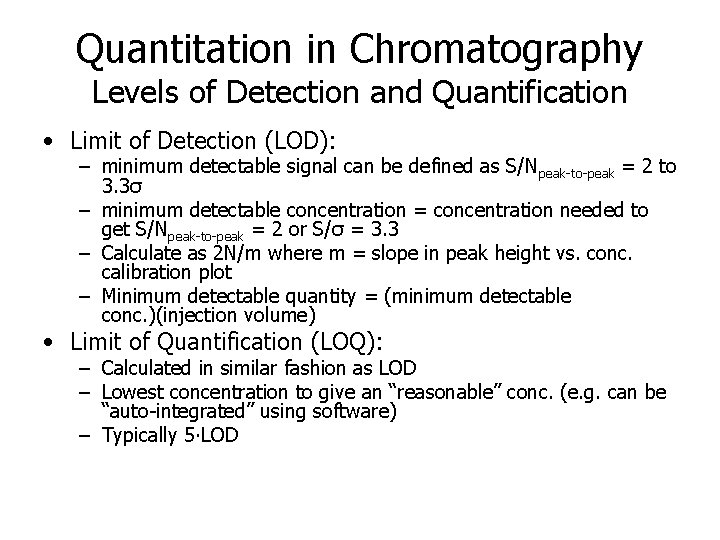
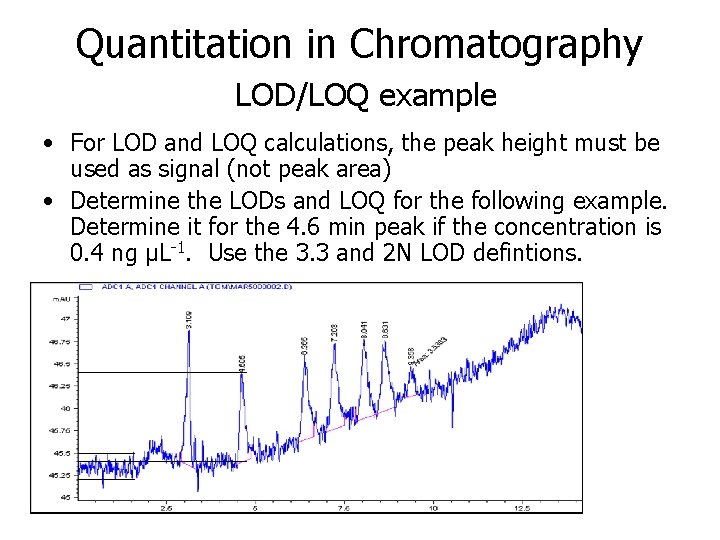
Quantitation in Chromatography Levels of Detection and Quantification • Limit of Detection (LOD): – minimum detectable signal can be defined as S/Npeak-to-peak = 2 to 3. 3σ – minimum detectable concentration = concentration needed to get S/Npeak-to-peak = 2 or S/σ = 3. 3 – Calculate as 2 N/m where m = slope in peak height vs. conc. calibration plot – Minimum detectable quantity = (minimum detectable conc. )(injection volume) • Limit of Quantification (LOQ): – Calculated in similar fashion as LOD – Lowest concentration to give an “reasonable” conc. (e. g. can be “auto-integrated” using software) – Typically 5∙LOD

Quantitation in Chromatography LOD/LOQ example • For LOD and LOQ calculations, the peak height must be used as signal (not peak area) • Determine the LODs and LOQ for the following example. Determine it for the 4. 6 min peak if the concentration is 0. 4 ng μL-1. Use the 3. 3 and 2 N LOD defintions.

Quantitation in Chromatography Calibration • Software for Calculations – Instrument Software (e. g. Chem. Station) • typical method used in industry • requires setting up all work before running standards • often allows checks of calibration quality before running samples – Excel • we will use here for a number of reasons (only have to learn one software program, example spreadsheets often available in texts, more data processing flexibility)

Quantitation in Chromatography Calibration • Calibration Methods – using Excel – External Standard – linear calibration • • Standards prepared Standards and Samples analyzed on same day Use Excel spreadsheet in HW Set 1, problem 3 as an example Also, see p. 301 -302 of Miller and p. 87 -91 of Harris (Quantitative Chemical Analysis) for examples – Non-linear Calibration • With some detectors (S – FPD and ELSD) or when operating at high concentrations, non-linear calibration curves are needed • Mostly can use Y = a + bx + cx 2 (2 nd order polynomial fit) or power fit (log. Y = b + mlog. C or Y = 10 b. Cm) methods • Statistics to find uncertainty is much more complex

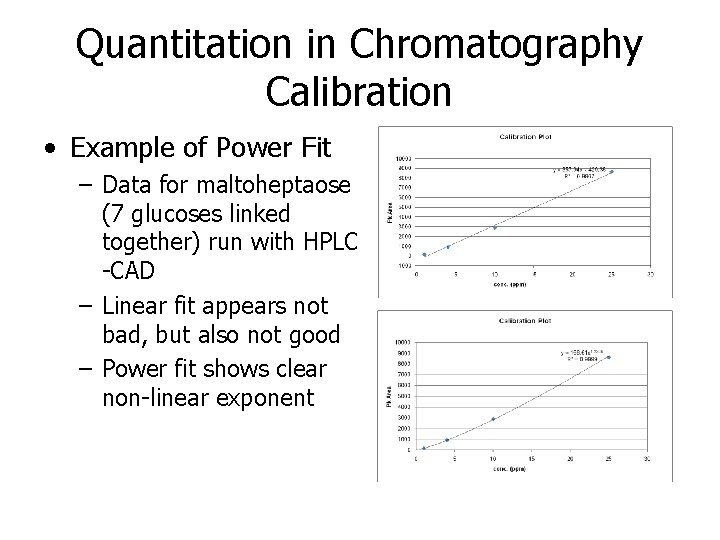
Quantitation in Chromatography Calibration • Example of Power Fit – Data for maltoheptaose (7 glucoses linked together) run with HPLC -CAD – Linear fit appears not bad, but also not good – Power fit shows clear non-linear exponent

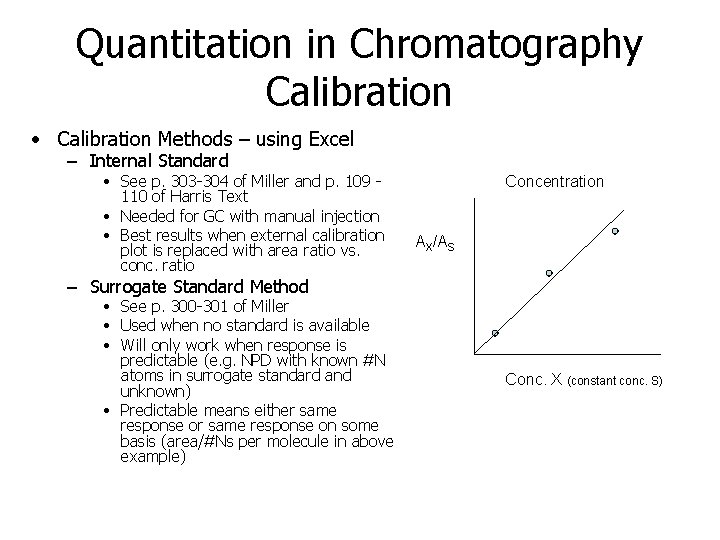
Quantitation in Chromatography Calibration • Calibration Methods – using Excel – Internal Standard • See p. 303 -304 of Miller and p. 109 110 of Harris Text • Needed for GC with manual injection • Best results when external calibration plot is replaced with area ratio vs. conc. ratio Concentration AX/AS – Surrogate Standard Method • See p. 300 -301 of Miller • Used when no standard is available • Will only work when response is predictable (e. g. NPD with known #N atoms in surrogate standard and unknown) • Predictable means either same response on some basis (area/#Ns per molecule in above example) Conc. X (constant conc. S)

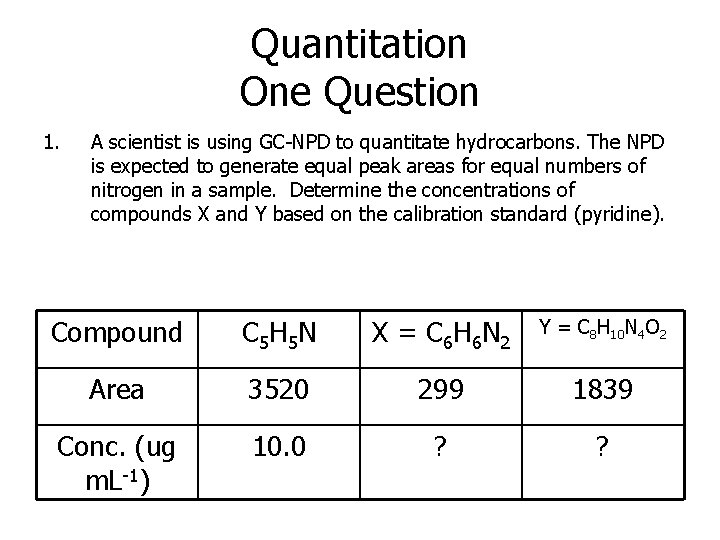
Quantitation One Question 1. A scientist is using GC-NPD to quantitate hydrocarbons. The NPD is expected to generate equal peak areas for equal numbers of nitrogen in a sample. Determine the concentrations of compounds X and Y based on the calibration standard (pyridine). Compound C 5 H 5 N X = C 6 H 6 N 2 Y = C 8 H 10 N 4 O 2 Area 3520 299 1839 Conc. (ug m. L-1) 10. 0 ? ?