Chem 231 211 Lecture Announcements I Return Homework








- Slides: 16

Chem. 231 – 2/11 Lecture

Announcements I • • Return Homework Set 1 Quiz 1 Today (15 min. ) New Homework Set (Set 2) Website Update – Homework 1 Solutions – Adding demonstration for HW 2

Announcements II • Today’s Topics: – Finish Extractions • Quantitative calculations • How to determine if method is working and how to improve methods – Low Performance Chromatography • Lower pressure chromatography • Thin layer chromatography – Quantitative Chromatography • Starting early • Focus today on integrating chromatograms

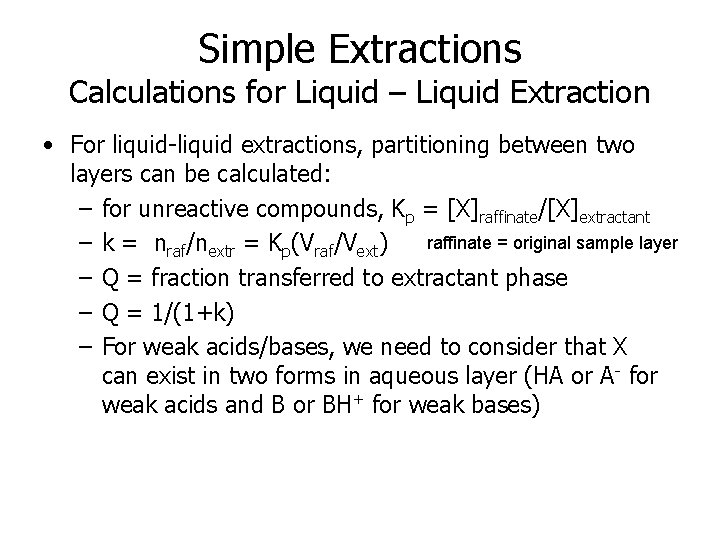
Simple Extractions Calculations for Liquid – Liquid Extraction • For liquid-liquid extractions, partitioning between two layers can be calculated: – for unreactive compounds, Kp = [X]raffinate/[X]extractant raffinate = original sample layer – k = nraf/nextr = Kp(Vraf/Vext) – Q = fraction transferred to extractant phase – Q = 1/(1+k) – For weak acids/bases, we need to consider that X can exist in two forms in aqueous layer (HA or A- for weak acids and B or BH+ for weak bases)

Simple Extractions Calculations for Liquid – Liquid Extraction • Sample Calculation for Butyric acid CH 3(CH 2)2 CO 2 H with KOW = 5. 75 and Ka = 4. 82. • Assuming an octanol raffinate phase, lets calculate fraction extracted to an aqueous phase as a function of p. H assuming 20 m. L aqueous phase and 10 m. L octanol • KD = [HA]total aq/[HA]Octanol and Kp = 5. 75 • Ka = [H+][A-]/[HA] = 10 -4. 82 = 1. 51 x 10 -5 • Since Kp = [HA]Oct/[HA]aq and KD = [HA]Oct/([A-] + [HA])aq, KD/Kp = [HA]aq/([A-] + [HA]) = a = nonionized fraction • a = [HA]/([A-] + [HA]) = [HA]/(Ka[HA]/[H+]+ [HA]) = [H+]/(Ka+ [H+]) = f(p. Ka, p. H) [note: different equation for weak bases] • KD = Kpa • k = Kpa(Voct/Vaq) • Q = 1/(1 + k) • Go to Excel Demonstration

Simple Extractions Calculations for Liquid – Liquid Extraction • For best separation, want high Q value for one compound and low Q value for other compound • Go to 3 -Mepyridine, 2 -naphthaleneamine separation

Simple Extractions Calculations for other methods (SPE) • Not Quantitative (too many variables) • Can Make Predictions about Relative Retention • Example: want to know if 2 -chlorophenol will be retained on SPE cartridge – If phenol has smaller KOW and has near 100% retention, 2 -chlorophenol should also be retained

Simple Extractions Tests • Numerous losses are possible: – inefficient retention – inefficient sample transfers – inefficient removal of trapped compounds • Best strategy is to test recovery (and for each step if recovery is low) • Small sample sizes and concentrations are challenging

Low Performance Chromatography Lower Pressure Chromatography • Purpose of Separation – Typically used for preparative chromatography – Commonly used when simple extractions don’t have sufficient resolution (e. g. removal of desired product from reactants and distinctly different side reaction products) • Equipment – – packed columns (usually normal phase) injection system or manual placement of sample flow provided by low pressure pump, gravity or gas pressure (Flash) means for fraction collection more common than means for detection • Advantages/Disadvantages – better separation than simple methods and lower cost than HPLC – limited resolution is main disadvantage

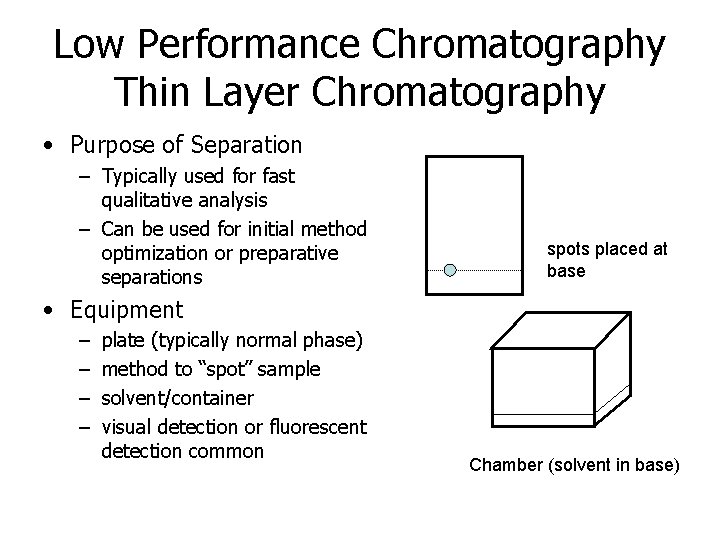
Low Performance Chromatography Thin Layer Chromatography • Purpose of Separation – Typically used for fast qualitative analysis – Can be used for initial method optimization or preparative separations spots placed at base • Equipment – – plate (typically normal phase) method to “spot” sample solvent/container visual detection or fluorescent detection common Chamber (solvent in base)

Low Performance Chromatography Thin Layer Chromatography • Advantages – – – relatively fast (especially considering start up time) low cost simple detection can run multiple samples in parallel whole sample seen (whether doesn’t migrate or moves with solvent) • Disadvantages – – not very quantitative limited sample size limited resolution not as reproducible

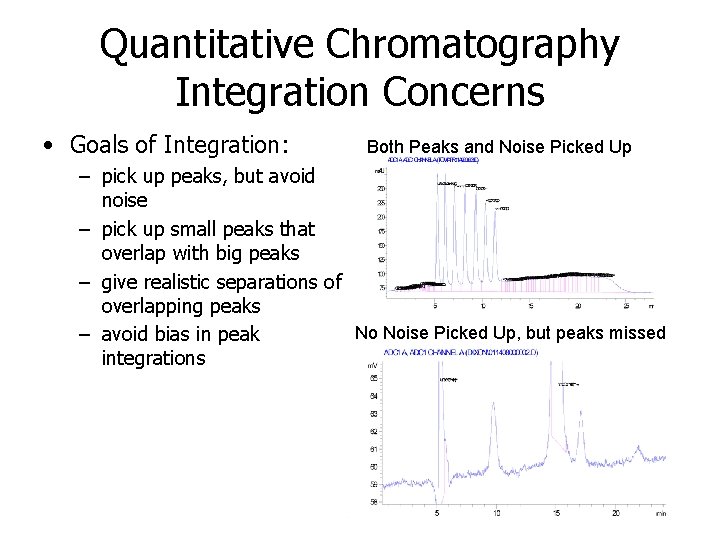
Quantitative Chromatography Integration Concerns • Goals of Integration: Both Peaks and Noise Picked Up – pick up peaks, but avoid noise – pick up small peaks that overlap with big peaks – give realistic separations of overlapping peaks No Noise Picked Up, but peaks missed – avoid bias in peak integrations

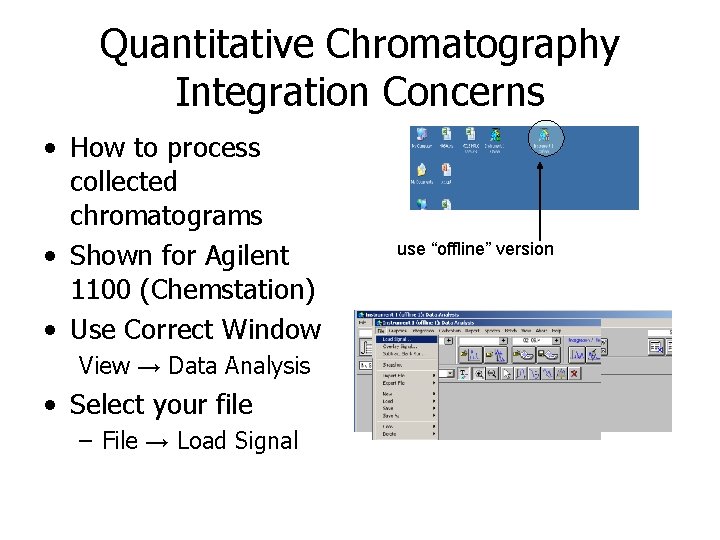
Quantitative Chromatography Integration Concerns • How to process collected chromatograms • Shown for Agilent 1100 (Chemstation) • Use Correct Window View → Data Analysis • Select your file – File → Load Signal use “offline” version

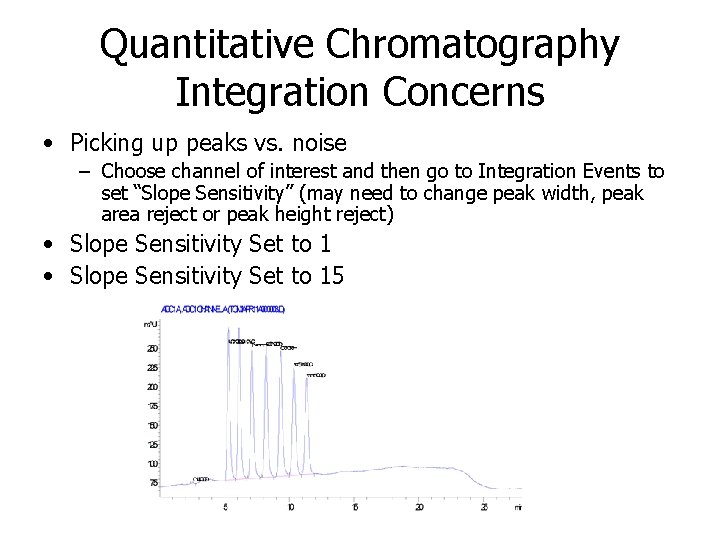
Quantitative Chromatography Integration Concerns • Picking up peaks vs. noise – Choose channel of interest and then go to Integration Events to set “Slope Sensitivity” (may need to change peak width, peak area reject or peak height reject) • Slope Sensitivity Set to 15

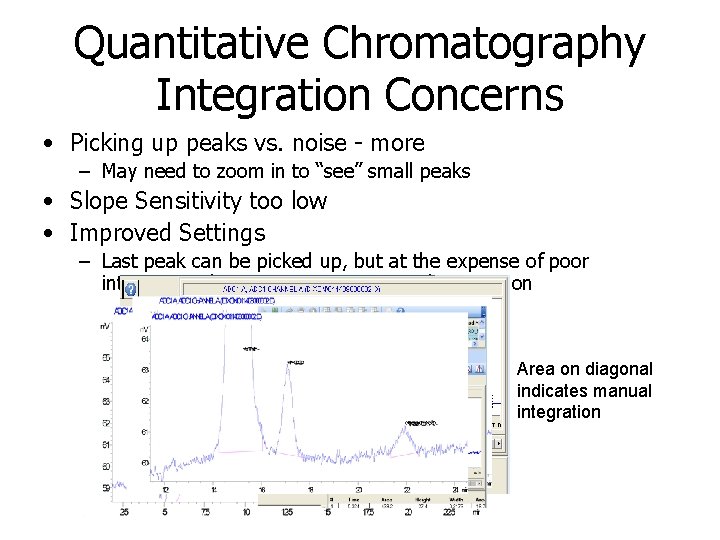
Quantitative Chromatography Integration Concerns • Picking up peaks vs. noise - more – May need to zoom in to “see” small peaks • Slope Sensitivity too low • Improved Settings – Last peak can be picked up, but at the expense of poor integration; better to just use manual integration Area on diagonal indicates manual integration

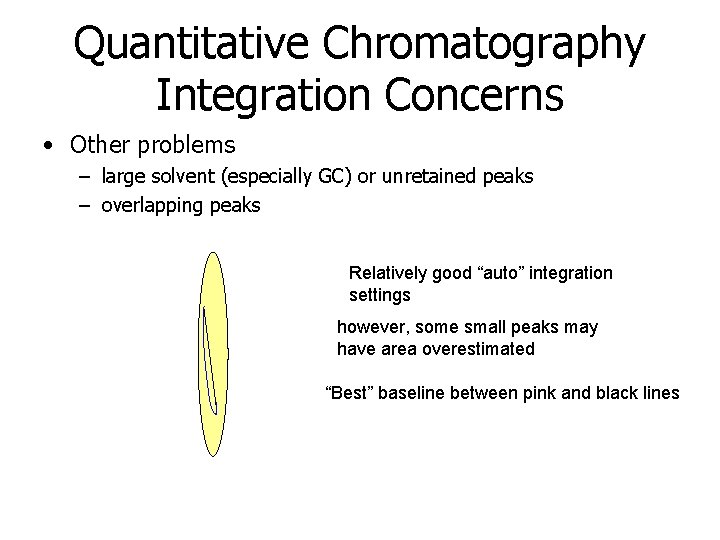
Quantitative Chromatography Integration Concerns • Other problems – large solvent (especially GC) or unretained peaks – overlapping peaks Relatively good “auto” integration settings however, some small peaks may have area overestimated “Best” baseline between pink and black lines