CENTRAL CONTROLS OF FOOD INTAKE AND APPETITE PartI





- Slides: 13

CENTRAL CONTROLS OF FOOD INTAKE AND APPETITE Part-I Dr Pramod Kumar Asstt. Professor Department of Veterinary Physiology Bihar Veterinary College, Patna

q q q q CENTRAL CONTROLS OF FOOD INTAKE AND APPETITE Coordination by the Hypothalamus Role of the Brainstem Neuropeptides Central Neurotransmitters Hedonic Mechanisms Mnemonic Representations of Experience with Food Endocannabinoids

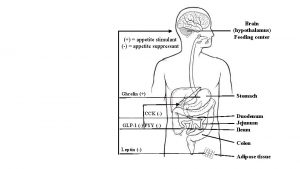
Coordination by the Hypothalamus - “gate keeper” in the control of food intake and appetite. Peripheral signals of energy balance may act directly on the hypothalamus to control food intake communication between the hypothalamus and higher cortical centers pertaining to food memory and rewarding aspects of food lateral hypothalamus “hunger center, ” medial hypothalamus “satiety center”.

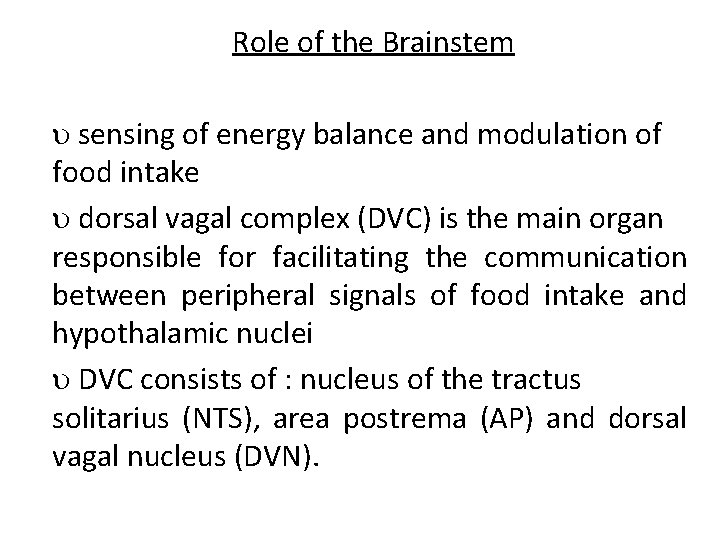
Role of the Brainstem sensing of energy balance and modulation of food intake dorsal vagal complex (DVC) is the main organ responsible for facilitating the communication between peripheral signals of food intake and hypothalamic nuclei DVC consists of : nucleus of the tractus solitarius (NTS), area postrema (AP) and dorsal vagal nucleus (DVN).

Vagal nerve afferents: carry sensory information relaying hunger and satiety from the gut directly to the NTS (increased meal size and duration) AP receive metabolic signals of energy balance (e. g. , hormones and nutrients carried by the blood) directly (absence of complete BBB) Efferent pathways : hypothalamus ↓→ DVN (modulates) ↓→ efferent vagal nerve activity → alter gastric emptying, gastric motility and pancreatic secretions.

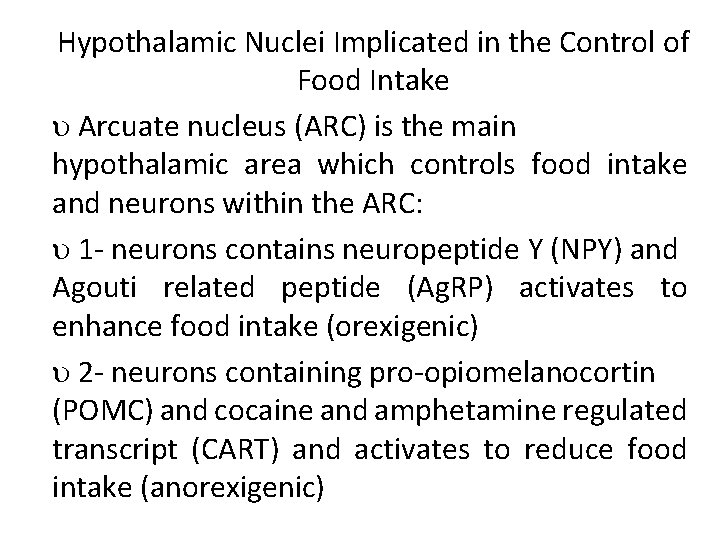
Hypothalamic Nuclei Implicated in the Control of Food Intake Arcuate nucleus (ARC) is the main hypothalamic area which controls food intake and neurons within the ARC: 1 - neurons contains neuropeptide Y (NPY) and Agouti related peptide (Ag. RP) activates to enhance food intake (orexigenic) 2 - neurons containing pro-opiomelanocortin (POMC) and cocaine and amphetamine regulated transcript (CART) and activates to reduce food intake (anorexigenic)

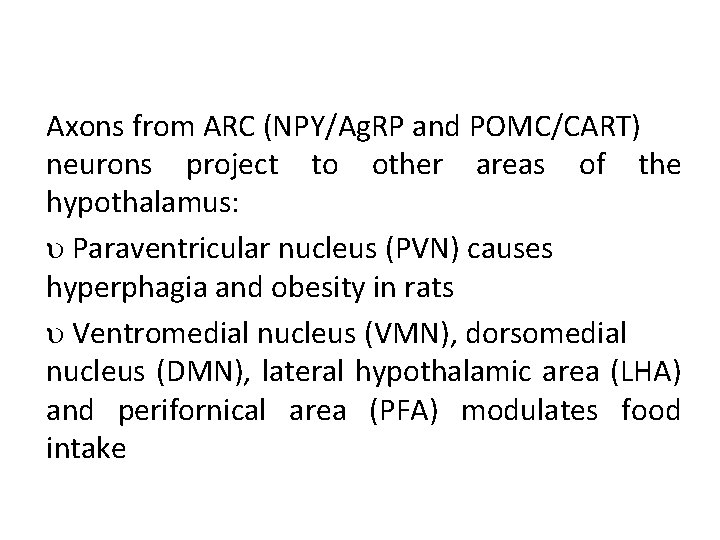
Axons from ARC (NPY/Ag. RP and POMC/CART) neurons project to other areas of the hypothalamus: Paraventricular nucleus (PVN) causes hyperphagia and obesity in rats Ventromedial nucleus (VMN), dorsomedial nucleus (DMN), lateral hypothalamic area (LHA) and perifornical area (PFA) modulates food intake

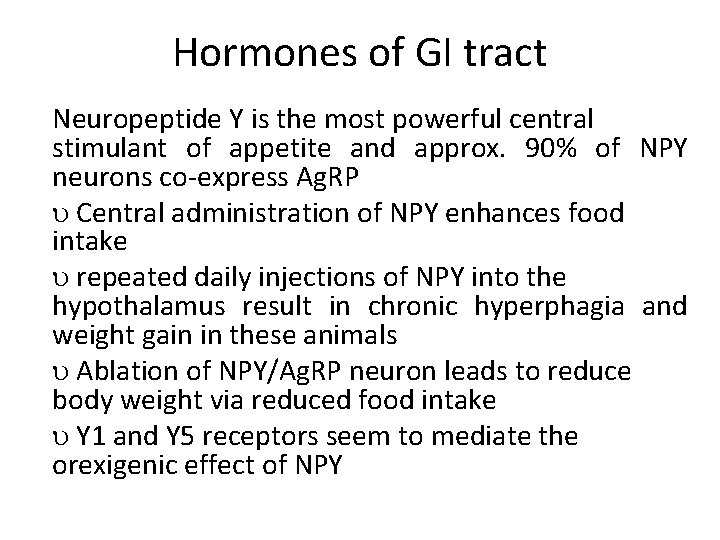
Hormones of GI tract Neuropeptide Y is the most powerful central stimulant of appetite and approx. 90% of NPY neurons co-express Ag. RP Central administration of NPY enhances food intake repeated daily injections of NPY into the hypothalamus result in chronic hyperphagia and weight gain in these animals Ablation of NPY/Ag. RP neuron leads to reduce body weight via reduced food intake Y 1 and Y 5 receptors seem to mediate the orexigenic effect of NPY

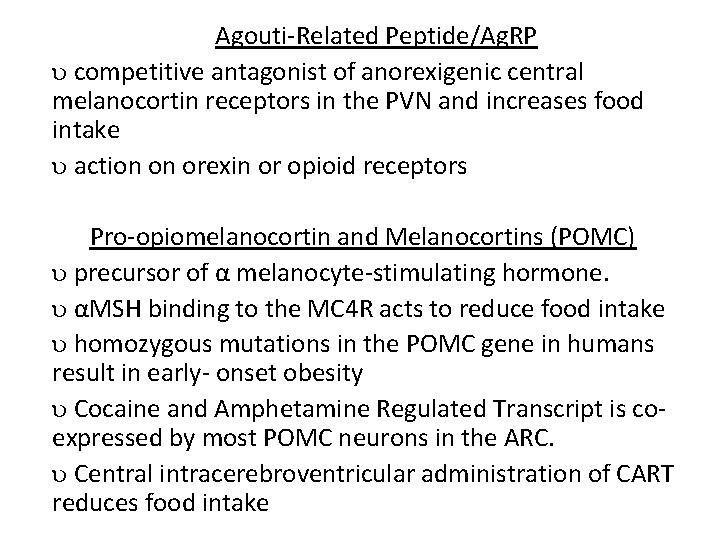
Agouti-Related Peptide/Ag. RP competitive antagonist of anorexigenic central melanocortin receptors in the PVN and increases food intake action on orexin or opioid receptors Pro-opiomelanocortin and Melanocortins (POMC) precursor of α melanocyte-stimulating hormone. αMSH binding to the MC 4 R acts to reduce food intake homozygous mutations in the POMC gene in humans result in early- onset obesity Cocaine and Amphetamine Regulated Transcript is coexpressed by most POMC neurons in the ARC. Central intracerebroventricular administration of CART reduces food intake

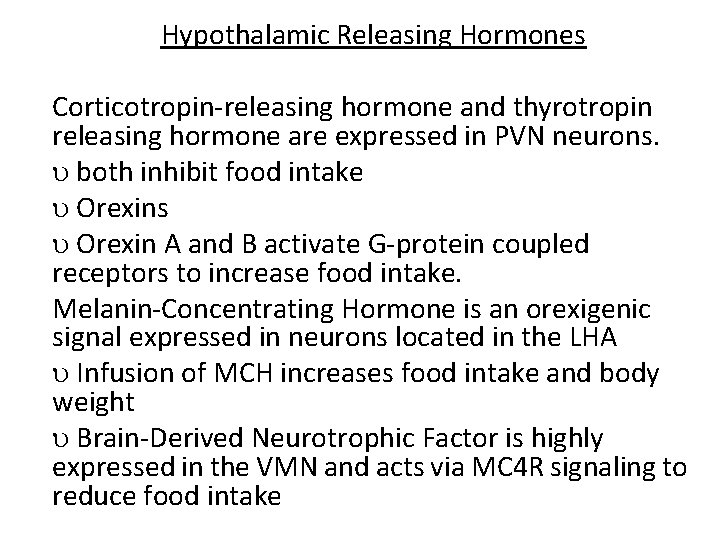
Hypothalamic Releasing Hormones Corticotropin-releasing hormone and thyrotropin releasing hormone are expressed in PVN neurons. both inhibit food intake Orexins Orexin A and B activate G-protein coupled receptors to increase food intake. Melanin-Concentrating Hormone is an orexigenic signal expressed in neurons located in the LHA Infusion of MCH increases food intake and body weight Brain-Derived Neurotrophic Factor is highly expressed in the VMN and acts via MC 4 R signaling to reduce food intake

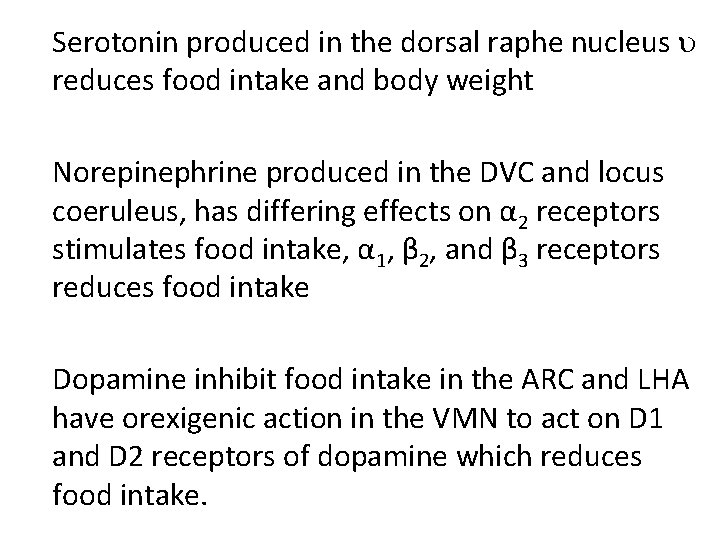
Serotonin produced in the dorsal raphe nucleus reduces food intake and body weight Norepinephrine produced in the DVC and locus coeruleus, has differing effects on α 2 receptors stimulates food intake, α 1, β 2, and β 3 receptors reduces food intake Dopamine inhibit food intake in the ARC and LHA have orexigenic action in the VMN to act on D 1 and D 2 receptors of dopamine which reduces food intake.

Hedonic mechanisms and cortico-limbic pathways control appetite and food intake Visual, smell and taste signals can override satiety signals to maintain food intake These sensory signals are conveyed from NTS in the brainstem to cortico-limbic reward centers implicated in appetite regulation Dopamine, serotonin, opioids and nor-epinephrine have been implicated as important neurotransmitters involved in signaling within this network. Mnemonic representations of experience with food – Past experience with specific foods forms an important contributor to continue consumption Orbito-frontal cortex (OFC), an area that receives converging sensory input in the non-homeostatic control of food intake.

Endocannabinoids shown to produce a dose dependent orexigenic effect and this effect is thought to occur via modulation of reward circuitry 1) Anandamide derived from membranous phospholipids 2) Arachidonoylglycerol (2 -AG), derived from triglycerides Endocannabinoids may also act directly on the hypothalamus to exert their orexigenic effect. These substances are secreted by postsynaptic neurons and act in retrograde fashion.
 General controls vs application controls
General controls vs application controls He who controls the past controls the future
He who controls the past controls the future Appetizers are food which stimulate the appetite
Appetizers are food which stimulate the appetite Diabetic diet chart pdf
Diabetic diet chart pdf Food safety controls
Food safety controls Food safety controls
Food safety controls Instinct emotion
Instinct emotion Salad that stimulate appetite
Salad that stimulate appetite Appetite approval ambition
Appetite approval ambition Risk of aspiration nursing diagnosis
Risk of aspiration nursing diagnosis Risk appetite pwc
Risk appetite pwc Appetite logo
Appetite logo Apetimen syrup fructose
Apetimen syrup fructose Malnutrition ppt
Malnutrition ppt