ASTRAL1 Study SOFVEL in genotype 1 2 4

- Slides: 9

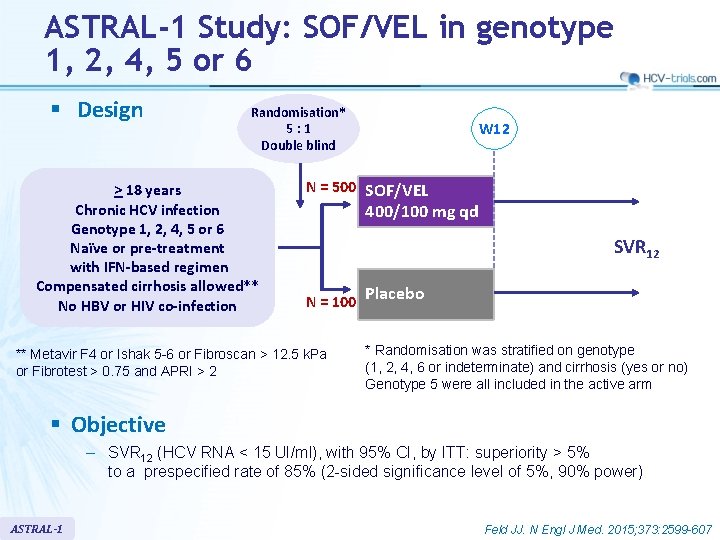
ASTRAL-1 Study: SOF/VEL in genotype 1, 2, 4, 5 or 6 § Design Randomisation* 5: 1 Double blind > 18 years Chronic HCV infection Genotype 1, 2, 4, 5 or 6 Naïve or pre-treatment with IFN-based regimen Compensated cirrhosis allowed** No HBV or HIV co-infection W 12 N = 500 SOF/VEL 400/100 mg qd SVR 12 N = 100 ** Metavir F 4 or Ishak 5 -6 or Fibroscan > 12. 5 k. Pa or Fibrotest > 0. 75 and APRI > 2 Placebo * Randomisation was stratified on genotype (1, 2, 4, 6 or indeterminate) and cirrhosis (yes or no) Genotype 5 were all included in the active arm § Objective – SVR 12 (HCV RNA < 15 UI/ml), with 95% CI, by ITT: superiority > 5% to a prespecified rate of 85% (2 -sided significance level of 5%, 90% power) ASTRAL-1 Feld JJ. N Engl J Med. 2015; 373: 2599 -607

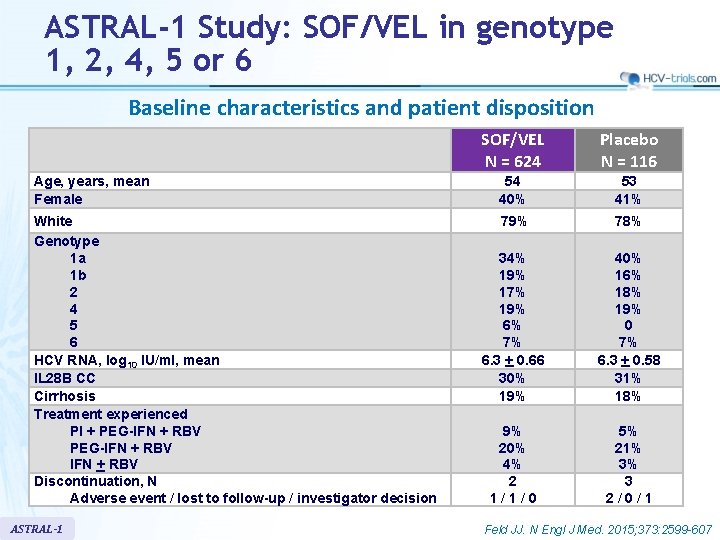
ASTRAL-1 Study: SOF/VEL in genotype 1, 2, 4, 5 or 6 Baseline characteristics and patient disposition SOF/VEL N = 624 Placebo N = 116 Age, years, mean Female 54 40% 53 41% White Genotype 1 a 1 b 2 4 5 6 HCV RNA, log 10 IU/ml, mean IL 28 B CC Cirrhosis Treatment experienced PI + PEG-IFN + RBV Discontinuation, N Adverse event / lost to follow-up / investigator decision 79% 78% 34% 19% 17% 19% 6% 7% 6. 3 + 0. 66 30% 19% 40% 16% 18% 19% 0 7% 6. 3 + 0. 58 31% 18% 9% 20% 4% 2 1/1/0 5% 21% 3% 3 2/0/1 ASTRAL-1 Feld JJ. N Engl J Med. 2015; 373: 2599 -607

ASTRAL-1 Study: SOF/VEL in genotype 1, 2, 4, 5 or 6 SVR 12 overall and by genotype, % (95 % CI) 100 % 100 100 99 * 99. 2 98. 1 97. 1 (96. 5 -100) (96. 9 -100) (91. 4 -100) (97. 9 - 99. 6) (95. 2 -99. 5) (95. 4 -100) (85. 1 -99. 9) 80 60 40 1 relapse 2 lost to follow-up 1 withdrew consent 0 1 death 1 relapse 20 624 210 118 104 116 35 41 Total 1 a 1 b 2 4 5 6 * Superiority to 85% (p < 0. 001) Genotype § SVR 12 according to baseline NS 5 A RAVs – Absent, N = 359, SVR 12 = 100% ; Present, N = 257, SVR 12 = 99. 2% ASTRAL-1 Feld JJ. N Engl J Med. 2015; 373: 2599 -607

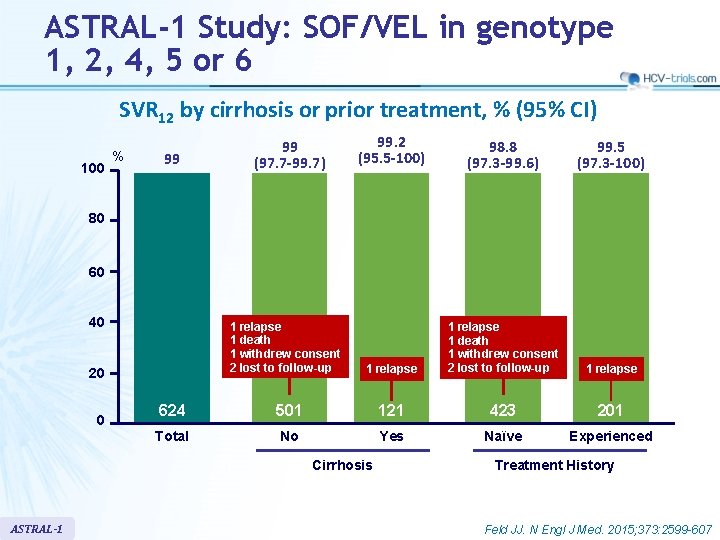
ASTRAL-1 Study: SOF/VEL in genotype 1, 2, 4, 5 or 6 SVR 12 by cirrhosis or prior treatment, % (95% CI) 100 % 99 99 (97. 7 -99. 7) 99. 2 (95. 5 -100) 98. 8 (97. 3 -99. 6) 99. 5 (97. 3 -100) 1 relapse 1 death 1 withdrew consent 2 lost to follow-up 1 relapse 80 60 40 1 relapse 1 death 1 withdrew consent 2 lost to follow-up 20 618 0 624 496 501501 120 121 Total No Yes Cirrhosis ASTRAL-1 418 423 Naïve 200 201 Experienced Treatment History Feld JJ. N Engl J Med. 2015; 373: 2599 -607

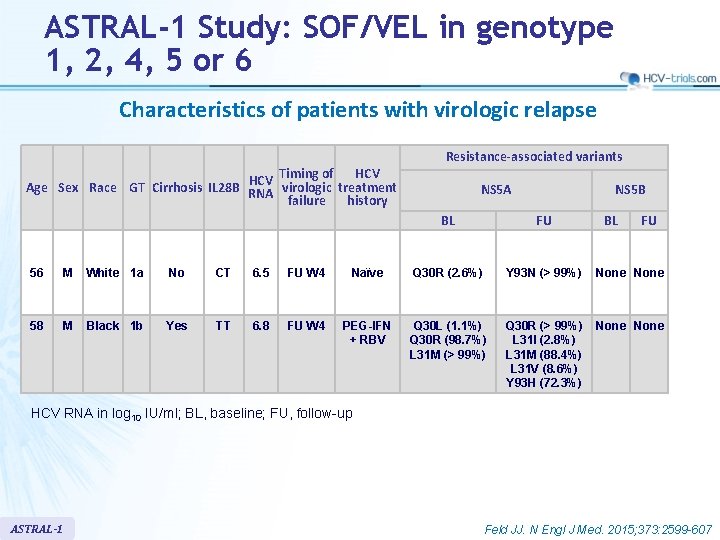
ASTRAL-1 Study: SOF/VEL in genotype 1, 2, 4, 5 or 6 Characteristics of patients with virologic relapse of HCV Timing Age Sex Race GT Cirrhosis IL 28 B RNA virologic treatment failure history Resistance-associated variants NS 5 A NS 5 B BL FU 56 M White 1 a No CT 6. 5 FU W 4 Naïve Q 30 R (2. 6%) Y 93 N (> 99%) None 58 M Black 1 b Yes TT 6. 8 FU W 4 PEG-IFN + RBV Q 30 L (1. 1%) Q 30 R (98. 7%) L 31 M (> 99%) Q 30 R (> 99%) L 31 I (2. 8%) L 31 M (88. 4%) L 31 V (8. 6%) Y 93 H (72. 3%) None HCV RNA in log 10 IU/ml; BL, baseline; FU, follow-up ASTRAL-1 Feld JJ. N Engl J Med. 2015; 373: 2599 -607

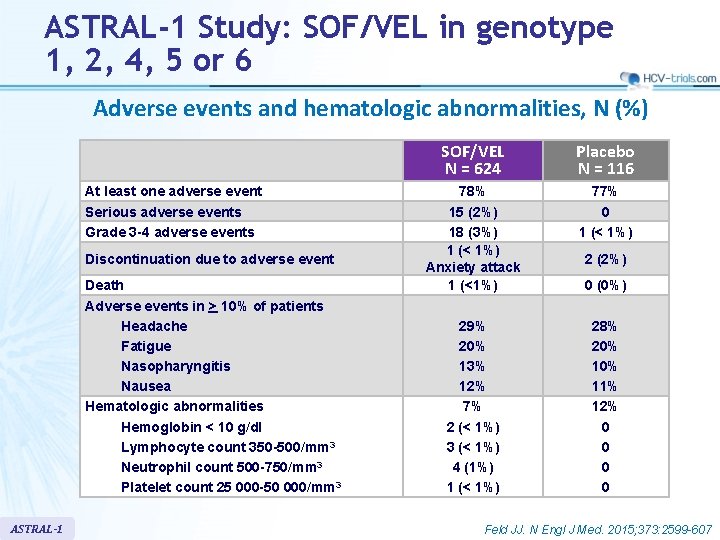
ASTRAL-1 Study: SOF/VEL in genotype 1, 2, 4, 5 or 6 Adverse events and hematologic abnormalities, N (%) At least one adverse event Serious adverse events Grade 3 -4 adverse events Discontinuation due to adverse event Death Adverse events in > 10% of patients Headache Fatigue Nasopharyngitis Nausea Hematologic abnormalities Hemoglobin < 10 g/dl Lymphocyte count 350 -500/mm 3 Neutrophil count 500 -750/mm 3 Platelet count 25 000 -50 000/mm 3 ASTRAL-1 SOF/VEL N = 624 Placebo N = 116 78% 15 (2%) 18 (3%) 1 (< 1%) Anxiety attack 1 (<1%) 77% 0 1 (< 1%) 29% 20% 13% 12% 7% 2 (< 1%) 3 (< 1%) 4 (1%) 1 (< 1%) 28% 20% 11% 12% 0 0 2 (2%) 0 (0%) Feld JJ. N Engl J Med. 2015; 373: 2599 -607

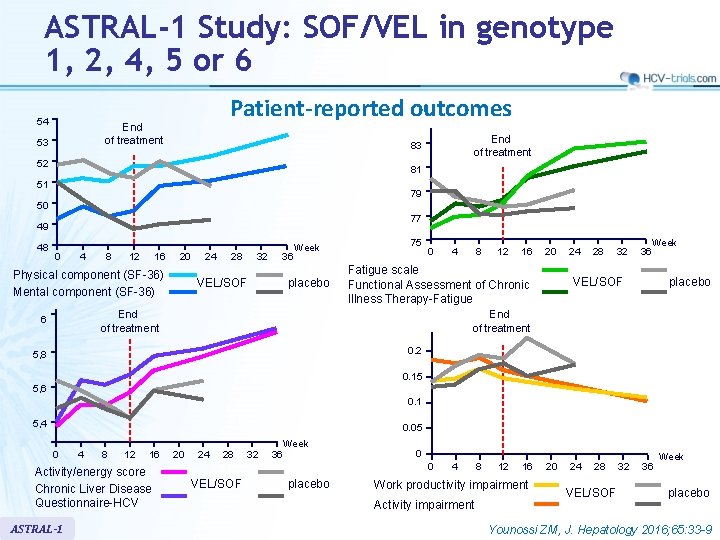
ASTRAL-1 Study: SOF/VEL in genotype 1, 2, 4, 5 or 6 54 Patient-reported outcomes End of treatment 53 End of treatment 83 52 81 51 79 50 77 49 48 0 4 8 12 16 20 Physical component (SF-36) Mental component (SF-36) 24 28 32 36 VEL/SOF Week placebo 75 0 4 12 16 20 Fatigue scale Functional Assessment of Chronic Illness Therapy-Fatigue End of treatment 6 8 24 28 32 Week 36 VEL/SOF placebo End of treatment 0. 2 5, 8 0. 15 5, 6 0. 1 5, 4 0. 05 0 4 8 12 16 Activity/energy score Chronic Liver Disease Questionnaire-HCV ASTRAL-1 20 24 28 32 36 Week 0 0 VEL/SOF placebo 4 8 12 16 Work productivity impairment Activity impairment 20 24 28 VEL/SOF 32 36 Week placebo Younossi ZM, J. Hepatology 2016; 65: 33 -9

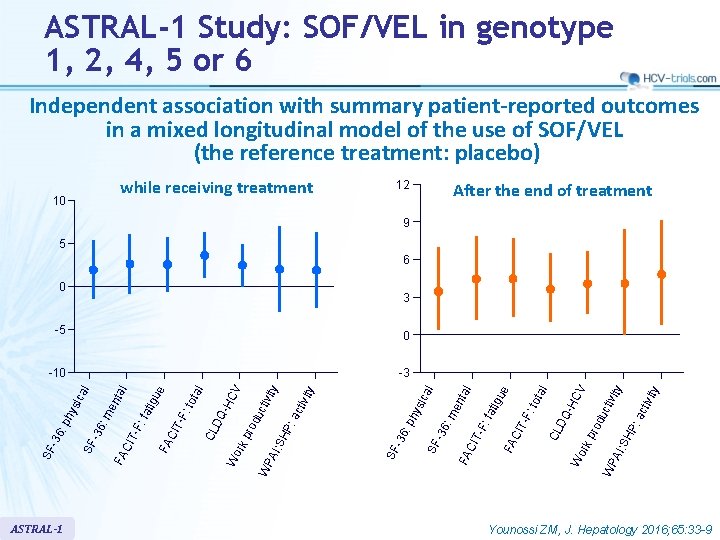
ASTRAL-1 Study: SOF/VEL in genotype 1, 2, 4, 5 or 6 Independent association with summary patient-reported outcomes in a mixed longitudinal model of the use of SOF/VEL (the reference treatment: placebo) while receiving treatment 10 12 After the end of treatment 9 5 6 0 : a ctiv ity ty SH P WP AI: pro du ctiv i CV Wo rk DQ -H l tot a CL FA CIT - F: fat i FA CIT -F: -36 SF hy : p -36 SF : m sic en tal al tivi ty ac ty WP AI: SH P: du ctiv i pro Wo rk CL DQ - tot a FA CIT -F: tig tal ITF: fa me n 6: SF -3 FA C ca hy si -36 : p SF ASTRAL-1 HC V -3 l -10 ue 0 l -5 gu e 3 Younossi ZM, J. Hepatology 2016; 65: 33 -9

ASTRAL-1 Study: SOF/VEL in genotype 1, 2, 4, 5 or 6 § Summary – In this international, randomized, double-blind, placebo-controlled phase III study, treatment with sofosbuvir–velpatasvir for 12 weeks resulted in high rates of SVR 12 (99%) in patients with HCV genotype 1, 2, 4, 5, or 6, including those with cirrhosis and those who had received previous treatment and those who had not been treated – Virologic failure was rare in patients infected with HCV genotype 1, and there were no virologic failures among those with HCV genotype 2, 4, 5 – Presence of baseline NS 5 A RAVs did not impact SVR 12 • Although the 2 patients who had a relapse had RAVs at baseline and at the time of virologic failure, 99% of the patients with baseline NS 5 A RAVs had a SVR 12, which suggests that pretreatment testing for RAVs is probably of little clinical value with SOF/VEL – Treatment with SOF/VEL for 12 weeks was well tolerated, with a safety profile similar to that of placebo treatment – SOF/VEL for 12 weeks provides a simple, safe, and highly effective treatment for patients with HCV GT 1, 2, 4, 5, or 6 infection, including those with compensated cirrhosis ASTRAL-1 Feld JJ. N Engl J Med. 2015; 373: 2599 -607