Aristotle an influential Greek philosopher 2500 years ago


- Slides: 10

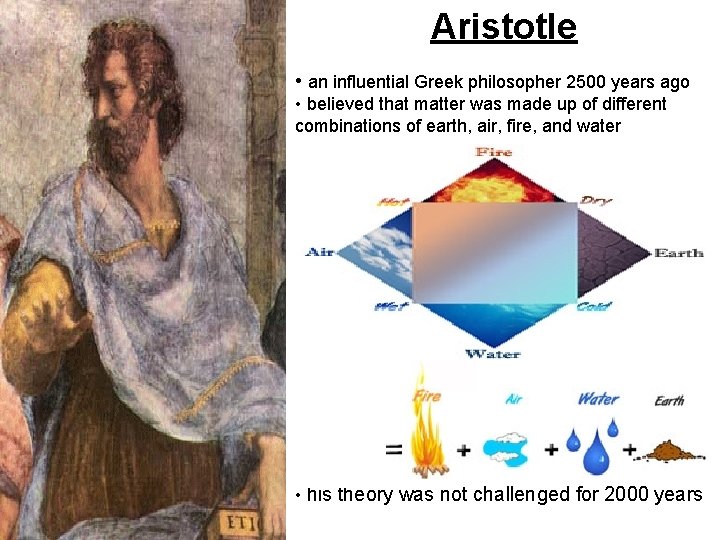
Aristotle • an influential Greek philosopher 2500 years ago • believed that matter was made up of different combinations of earth, air, fire, and water • his theory was not challenged for 2000 years

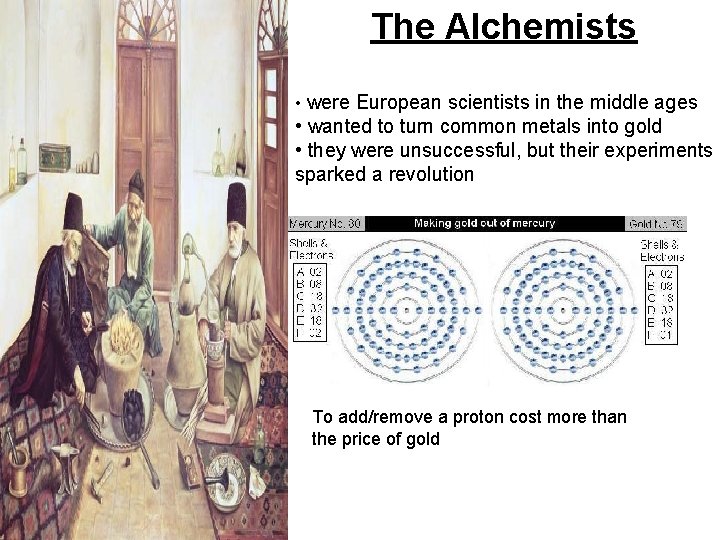
The Alchemists • were European scientists in the middle ages • wanted to turn common metals into gold • they were unsuccessful, but their experiments sparked a revolution To add/remove a proton cost more than the price of gold

John Dalton • • was a British school teacher (1766 -1844) credited for developing the Atomic Theory… 1. All matter is made of small particles called atoms 2. Atoms cannot be created, destroyed, or divided into smaller particles 3. All atoms of the same element are identical in mass and size, but they are different in mass and size from the atoms of other elements 4. Compounds are created when atoms of different elements link together in definite proportions

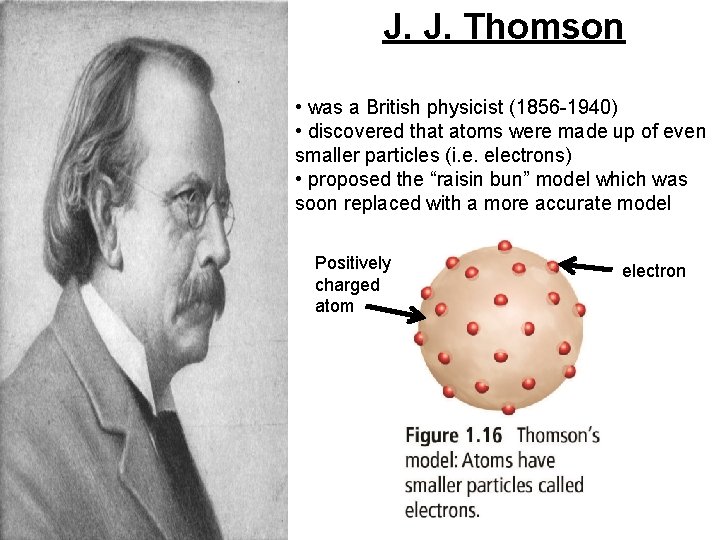
J. J. Thomson • was a British physicist (1856 -1940) • discovered that atoms were made up of even smaller particles (i. e. electrons) • proposed the “raisin bun” model which was soon replaced with a more accurate model Positively charged atom electron

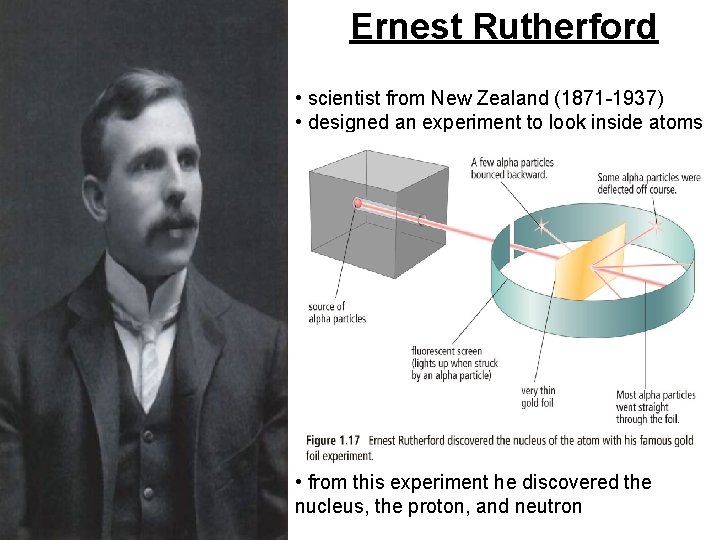
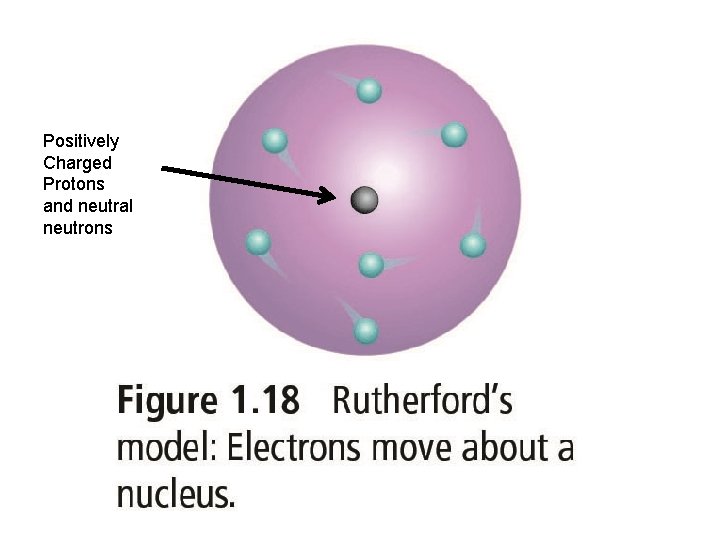
Ernest Rutherford • scientist from New Zealand (1871 -1937) • designed an experiment to look inside atoms • from this experiment he discovered the nucleus, the proton, and neutron

Positively Charged Protons and neutral neutrons

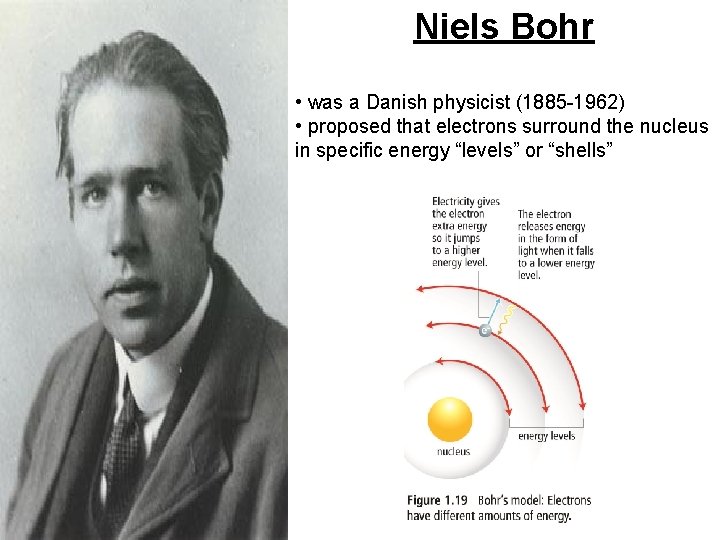
Niels Bohr • was a Danish physicist (1885 -1962) • proposed that electrons surround the nucleus in specific energy “levels” or “shells”

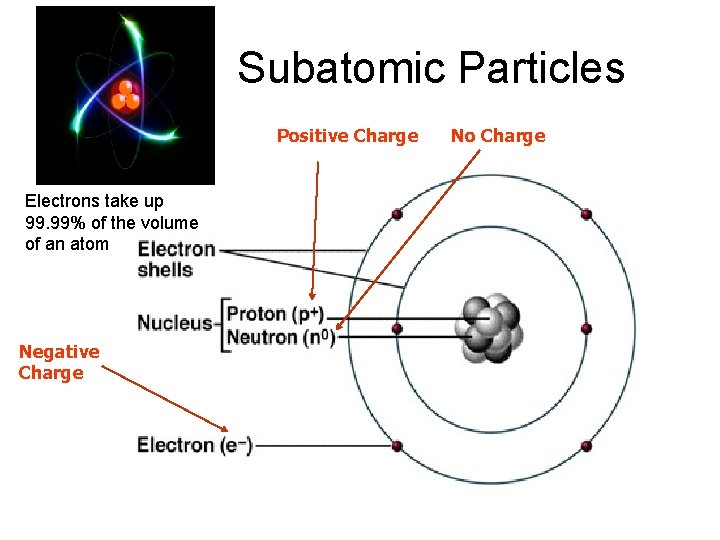
Subatomic Particles Positive Charge Electrons take up 99. 99% of the volume of an atom Negative Charge No Charge

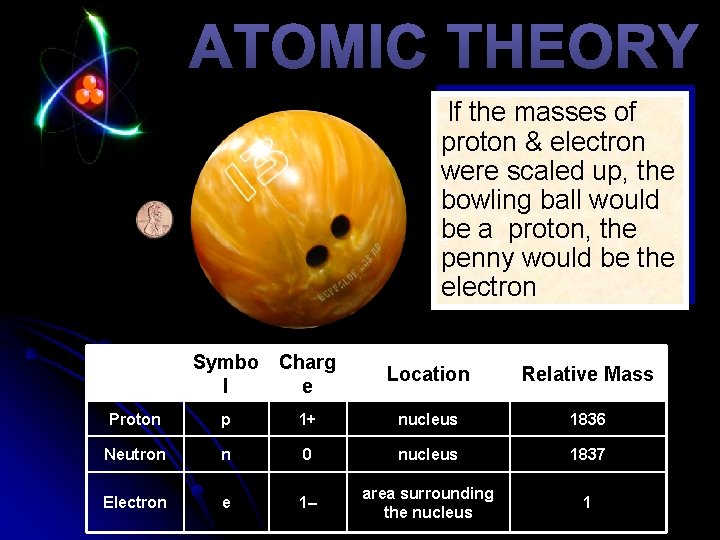
ATOMIC THEORY If the masses of proton & electron were scaled up, the bowling ball would be a proton, the penny would be the electron Symbo Charg l e Location Relative Mass Proton p 1+ nucleus 1836 Neutron n 0 nucleus 1837 Electron e 1– area surrounding the nucleus 1

Subatomic Particles (electron, proton and neutron) A proton, neutron, and electron went out to dinner one night. After a luxurious meal, the waiter brought the check to the proton and the electron. The neutron was perplexed as to why the waiter didn't bring him his check. So, he summoned the waiter to the table and asked him about it. The waiter explained to the neutron, "For you, there's no charge!” Why do all the other subatomic particles hate the electrons - they are so negative A proton walks into a bar and asks for a glass of water. The bartender asks, "are you sure you don't want root beer? " The proton replies, "I'm positive!
 Aristotle was a student of
Aristotle was a student of How long is four score and seven years
How long is four score and seven years Greek philosopher plato teacher
Greek philosopher plato teacher Which ancient greek philosopher defined tragedy?
Which ancient greek philosopher defined tragedy? The greek philosopher democritus
The greek philosopher democritus Which greek philosopher defined the art of persuasion
Which greek philosopher defined the art of persuasion What greek philosopher named the appeals ethos pathos logos
What greek philosopher named the appeals ethos pathos logos Shenzhen 30 years ago
Shenzhen 30 years ago You can easily forget how different life was 50 years ago
You can easily forget how different life was 50 years ago Four score and seven years ago our fathers
Four score and seven years ago our fathers Australia 50 000 years ago
Australia 50 000 years ago