ANJUMAN COLLEGE OF ENGINEERING TECHNOLOGY Sadar Nagpur DEPARTMENT


















- Slides: 26

ANJUMAN COLLEGE OF ENGINEERING & TECHNOLOGY Sadar, Nagpur

DEPARTMENT OF MECHANICAL ENGINEERING

A Presentation on “PROPERTIES OF STEAM” Name of Subject Teacher: Prof. Nafees P. Khan Name of Subject: Engg. Thermodynamics

CO attended CO 402 T. 4: Relate various steam properties, and analyze the various types of processes using steam as working fluid to determine the energy transfer in terms of heat and work.

Contents Ø Basic terms Ø Steam Thermodynamics Ø P–V Diagram, T–s Diagram, h–s Diagram and P–s Diagram Ø Steam Generation Ø Steam types Ø Measurement of dryness fraction of steam Ø Mollar’s diagram (h-s chart) Ø References

Basic terms • Steam Enthalpy (h) Enthalpy is a general measure of the internal stored energy per mass unit of a flowing stream. It is expressed in k. J/Kg (SI units). It is usually represented with letter h. • Steam Entropy (S) Entropy, is a measure of thermodynamic potential of a flowing stream in the units of energy per mass unit and absolute temperature. It is expressed in k. J/kg. K (SI units). It is usually represented with letter S. Specific Volume (v) As its name implies, specific volume is a measure of the volume of a flowing stream per mass unit. It is expressed in m 3/kg (SI units). It is usually represented with letter v.

• Sensible Heat: The amount of heat required to increase the temperature of 1 kg of water from 0 o. C to saturation temperature at a given pressure is known a s sensible heat of water. • Latent Heat The amount of heat required to convert 1 kg of water at saturation temperature for a given pressure into dry saturated steam at that temperature and pressure is known as latent heat of vaporization. • Dryness Fraction: Dryness fraction is defined as the mass of dry steam per kg of wet steam. It is represented by x. x = ms / (ms + mw), where: ms is the mass of steam and mw is the mass of water

Steam Thermodynamics • Steam is the gaseous phase of water. It utilizes • heat during the process and carries large • quantities of heat later. • Hence, it could be used as a working substance for steam engines. Steam is generated in boilers at constant pressure. • Generally, steam may be obtained starting from ice or straight away from the water by adding heat to it.

Under superheated or subcooled conditions, properties such as enthalpy, entropy and specific volume are dependent on temperature and pressure. However, at saturated conditions where a mixture of steam and water coexist, an additional parameter, steam quality, needs to be defined. Steam quality (i. e. dryness fraction), usually represented with letter x. Steam quality is frequently recorded as a percentage of steam by weight after being multiplied by 100% The mixture enthalpy, entropy and specific volume of a steamwater mixture can then be easily defined as follows: h = hf + x * hfg where, hfg= (hg-hf) S = sf + x* sfg = (sg-sf) v = vf + x*vg where subscripts f (from fluid) and g (from gas) refer to properties at saturated liquid and vapour conditions respectively.

P–V Diagram, T–s Diagram,

h–s Diagram and P–s Diagram

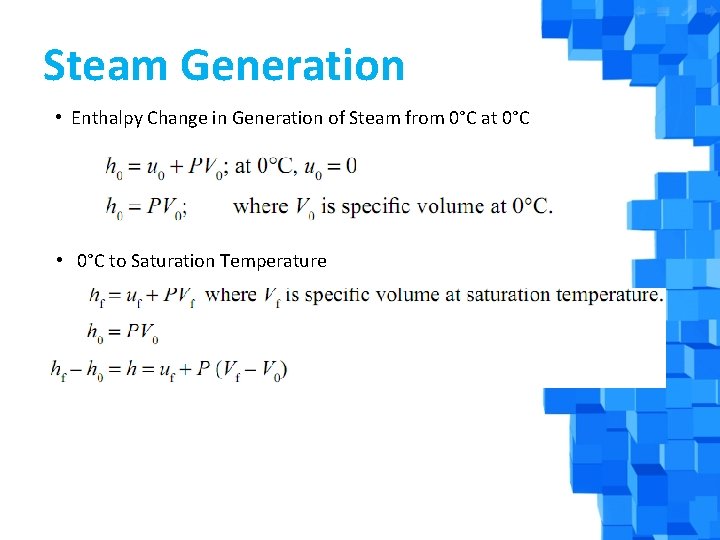
Steam Generation • Enthalpy Change in Generation of Steam from 0°C at 0°C • 0°C to Saturation Temperature

• Entropy of Steam


Steam Types Wet Steam: Wet steam contains partly water as suspended in it and partly steam Dry saturated Steam A steam at the saturation temprature corresponding to a given pressure and having no water molecules in it is known as dry saturated steam or dry steam. Since the dry saturated steam does not contain any water molecules in it, its dryness fraction will be unity. Superheated steam: When a dry saturated steam is heated further at the given constant pressure, its temperature rise beyond its saturation temperature. The steam in this state is said to be superheated.

Enthalpy of Steam 1. Enthalpy of liquid: hf=Cpw (tf-0) 2. Enthalpy of Dry saturated steam: hg=hf + hfg 3. Enthalpy of Wet steam: h=hf + x. hfg 4. Enthalpy of Superheated steam: hsup= hg+ Cps(Tsup –Tsat)

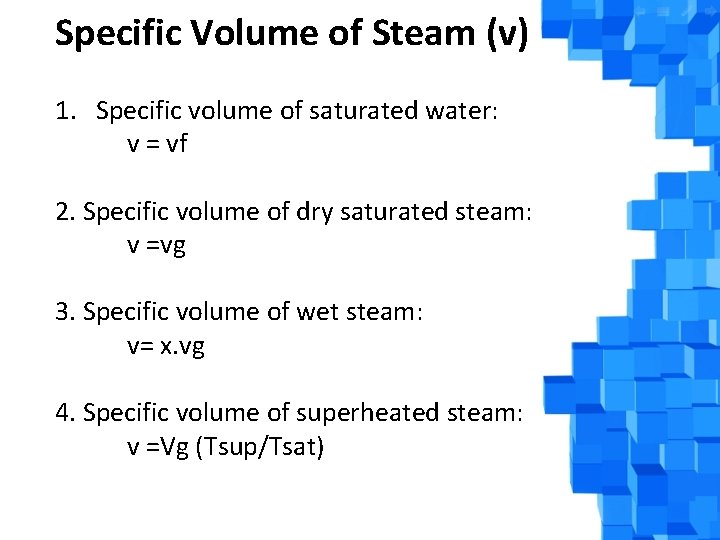
Specific Volume of Steam (v) 1. Specific volume of saturated water: v = vf 2. Specific volume of dry saturated steam: v =vg 3. Specific volume of wet steam: v= x. vg 4. Specific volume of superheated steam: v =Vg (Tsup/Tsat)

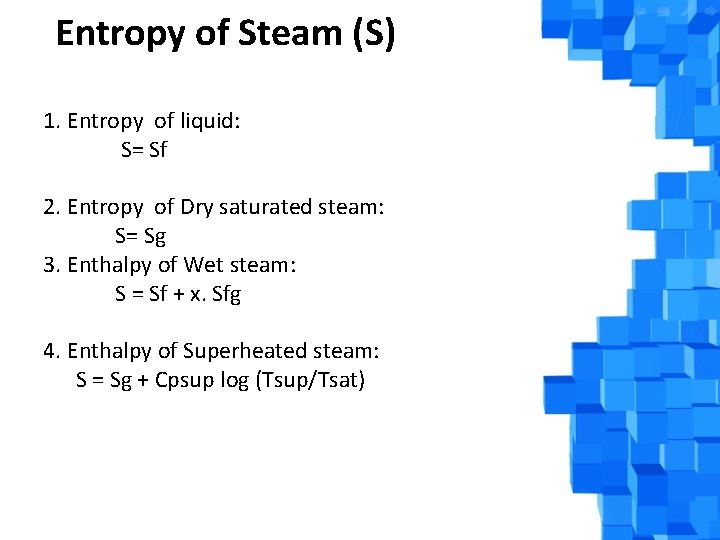
Entropy of Steam (S) 1. Entropy of liquid: S= Sf 2. Entropy of Dry saturated steam: S= Sg 3. Enthalpy of Wet steam: S = Sf + x. Sfg 4. Enthalpy of Superheated steam: S = Sg + Cpsup log (Tsup/Tsat)

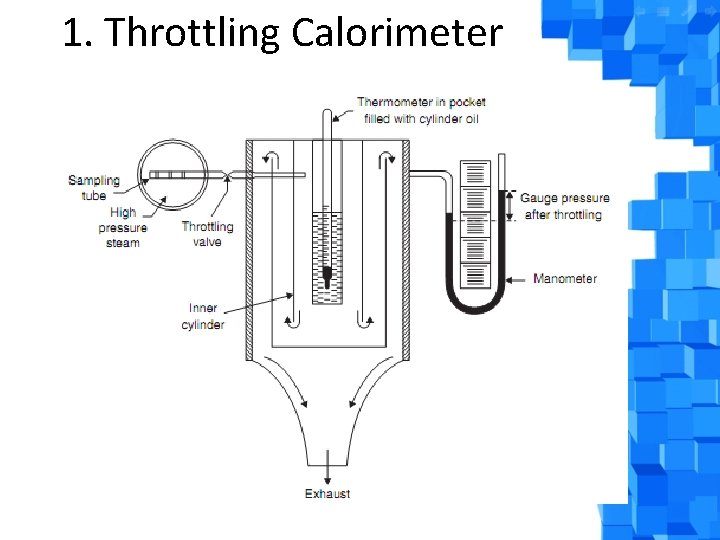
Measurement of dryness fraction (x) of steam by: 1. Throttling calorimeter 2. Separating and Throttling Calorimeter

1. Throttling Calorimeter


2. Separating and Throttling Calorimeter


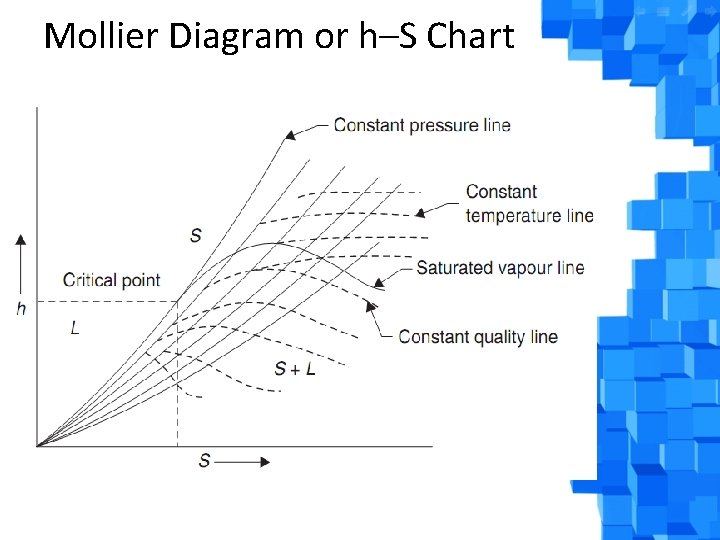
Mollier Diagram or h–S Chart


References 1. Engineering Thermodynamics, P. K. Nag, Tata Mc. Graw-Hill Publications 2. Engineering Thermodynamics, S. S. Khandare, Charotar Publication House 3. Thermal Engineering, P. L. Ballani, Khanna Publications 4. https: //www. ohio. edu/mechanical/thermo/property_tab es/H 2 O/ 5. https: //www. engineeringtoolbox. com/saturated-steam-properties d 273. html 6. http: //www. uniklinger. com/ask-the-expert/steam-engineering-guideline/basic -of-steam/properties-of-steam/
 The silenced by nadia anjuman
The silenced by nadia anjuman Manajemen risiko
Manajemen risiko 6 elemen budaya sadar risiko
6 elemen budaya sadar risiko Keluarga sadar gizi
Keluarga sadar gizi Contoh kasus culpa
Contoh kasus culpa Rela berkorban nyawa
Rela berkorban nyawa Usaha sadar adalah
Usaha sadar adalah Thakur college of engineering and technology
Thakur college of engineering and technology Fnu engineering courses
Fnu engineering courses Parasitic food chain example
Parasitic food chain example Swedish college of engineering and technology
Swedish college of engineering and technology Visvesvaraya technological university nagpur
Visvesvaraya technological university nagpur Public health institute nagpur
Public health institute nagpur Mount litera zee school besa nagpur fees structure
Mount litera zee school besa nagpur fees structure Ddu gky nagpur
Ddu gky nagpur Classification of nagpur road plan
Classification of nagpur road plan Nagpur garment industry
Nagpur garment industry Classification of roads by nagpur road plan
Classification of roads by nagpur road plan Nmc water bill nagpur
Nmc water bill nagpur Oklahoma alternative placement program
Oklahoma alternative placement program Department of information technology
Department of information technology Principal certification oklahoma
Principal certification oklahoma Department of electronics & information technology
Department of electronics & information technology E & it department odisha
E & it department odisha Pasadena city college police department
Pasadena city college police department Electrical engineering department
Electrical engineering department Engineering department in a hotel
Engineering department in a hotel