Alkanes Alkenes and Polymers WJEC Chemistry Unit 2

- Slides: 7

Alkanes, Alkenes and Polymers WJEC Chemistry Unit 2 - 2. 5 (part) WJEC Double Award Unit 5 - 5. 5 (part)

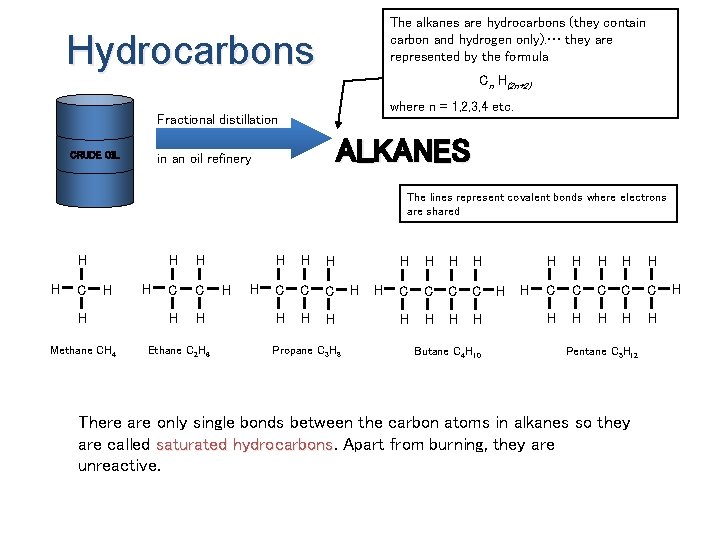
The alkanes are hydrocarbons (they contain carbon and hydrogen only). … they are represented by the formula Hydrocarbons Cn H(2 n+2) where n = 1, 2, 3, 4 etc. Fractional distillation CRUDE OIL ALKANES in an oil refinery The lines represent covalent bonds where electrons are shared H H C H H Methane CH 4 H H H C C H H Ethane C 2 H 6 H H H C C C H H H Propane C 3 H 8 H H H C C H H Butane C 4 H 10 H H H H C C C H H H Pentane C 5 H 12 There are only single bonds between the carbon atoms in alkanes so they are called saturated hydrocarbons Apart from burning, they are unreactive. H

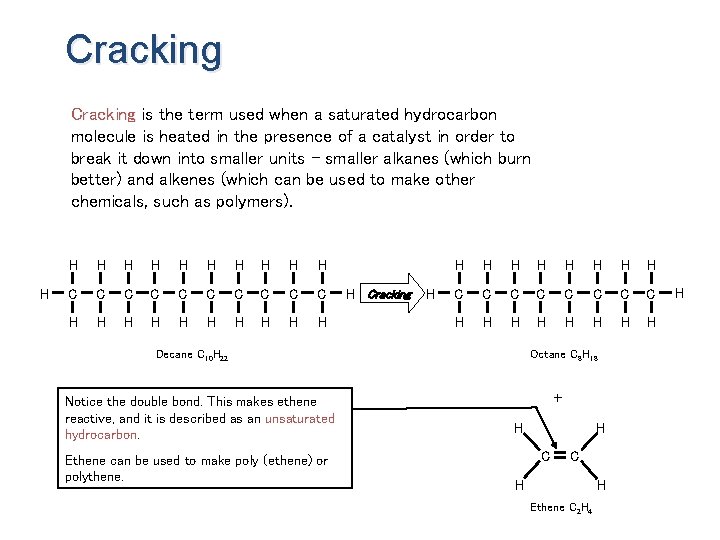
Cracking is the term used when a saturated hydrocarbon molecule is heated in the presence of a catalyst in order to break it down into smaller units – smaller alkanes (which burn better) and alkenes (which can be used to make other chemicals, such as polymers). H H H C C C C C H H H Cracking H H H H H C C C C H H H H Decane C 10 H 22 Notice the double bond. This makes ethene reactive, and it is described as an unsaturated hydrocarbon. Ethene can be used to make poly (ethene) or polythene. Octane C 8 H 18 + H H C C H H Ethene C 2 H 4 H

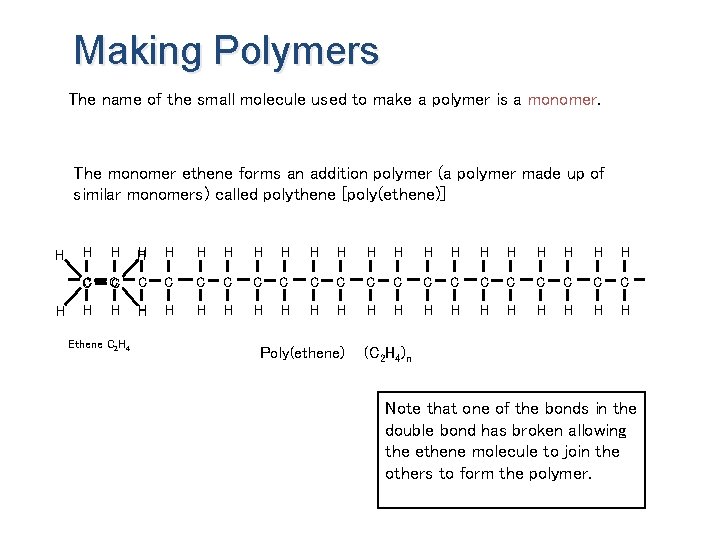
Making Polymers The name of the small molecule used to make a polymer is a monomer. The monomer ethene forms an addition polymer (a polymer made up of similar monomers) called polythene [poly(ethene)] H H H H H H C C C C C H H H H H Ethene C 2 H 4 Poly(ethene) (C 2 H 4)n Note that one of the bonds in the double bond has broken allowing the ethene molecule to join the others to form the polymer.

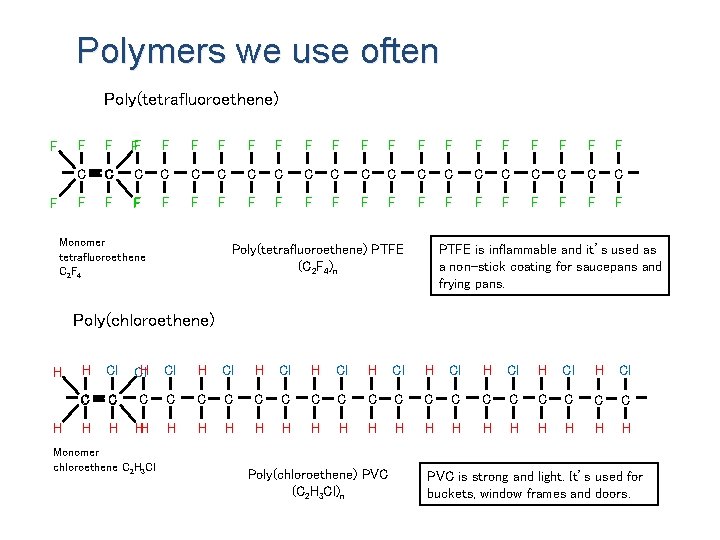
Polymers we use often Poly(tetrafluoroethene) F F FF F F F F C C C C C F F F F F F Monomer tetrafluoroethene C 2 F 4 Poly(tetrafluoroethene) PTFE (C 2 F 4)n PTFE is inflammable and it’s used as a non-stick coating for saucepans and frying pans. Poly(chloroethene) H H H Cl Cl. H Cl H Cl H Cl C C C C C H H H H H H Monomer chloroethene C 2 H 3 Cl Poly(chloroethene) PVC (C 2 H 3 Cl)n PVC is strong and light. It’s used for buckets, window frames and doors.

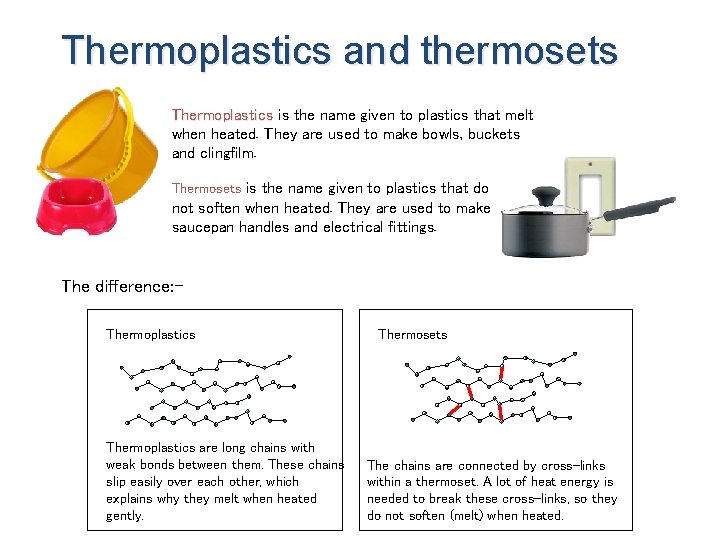
Thermoplastics and thermosets Thermoplastics is the name given to plastics that melt when heated. They are used to make bowls, buckets and clingfilm. Thermosets is the name given to plastics that do not soften when heated. They are used to make saucepan handles and electrical fittings. The difference: Thermoplastics are long chains with weak bonds between them. These chains slip easily over each other, which explains why they melt when heated gently. Thermosets The chains are connected by cross-links within a thermoset. A lot of heat energy is needed to break these cross-links, so they do not soften (melt) when heated.

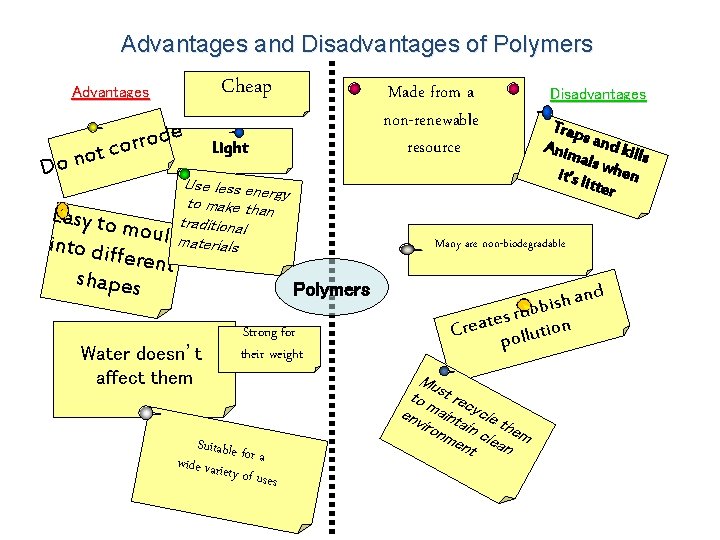
Advantages and Disadvantages of Polymers Cheap Advantages e d o r r o ot c n o D Made from a non-renewable resource Light Use less ene rgy to make than Easy to mouldtraditional materials into di fferent shapes Strong for their weight Suitable f wide varie or a ty of uses Trap s Anim and kill s als w h en it’s li tter Many are non-biodegradable Polymers Water doesn’t affect them Disadvantages h an s i b b ru s e t a on i Cre t u l l po Mu to st rec env maint ycle iron ain the me clea m nt n d












