A CLOSER LOOK AT THE ELEMENTS 92 elements



- Slides: 13

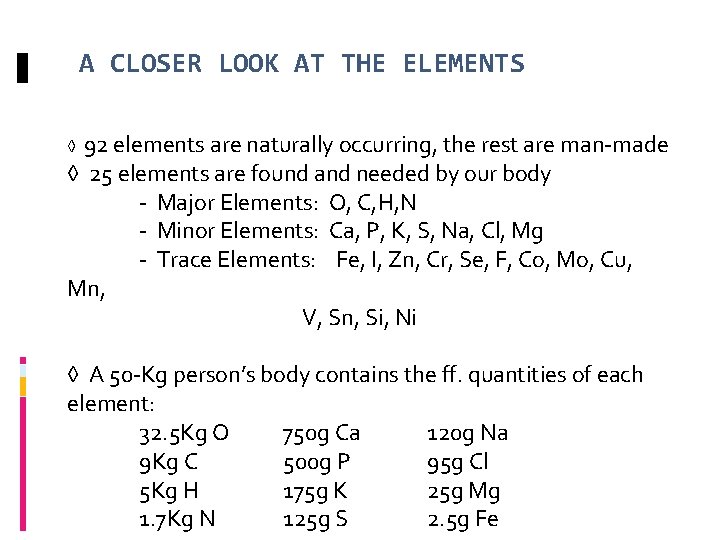
A CLOSER LOOK AT THE ELEMENTS 92 elements are naturally occurring, the rest are man-made ◊ 25 elements are found and needed by our body - Major Elements: O, C, H, N - Minor Elements: Ca, P, K, S, Na, Cl, Mg - Trace Elements: Fe, I, Zn, Cr, Se, F, Co, Mo, Cu, Mn, V, Sn, Si, Ni ◊ ◊ A 50 -Kg person’s body contains the ff. quantities of each element: 32. 5 Kg O 750 g Ca 120 g Na 9 Kg C 500 g P 95 g Cl 5 Kg H 175 g K 25 g Mg 1. 7 Kg N 125 g S 2. 5 g Fe

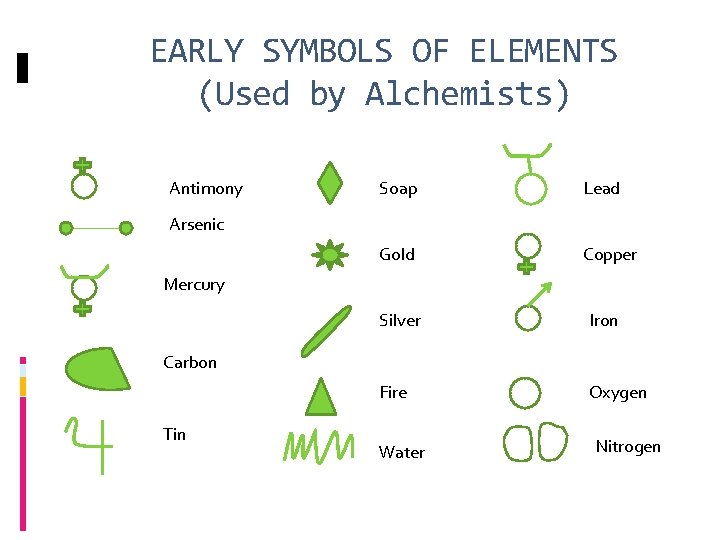
EARLY SYMBOLS OF ELEMENTS (Used by Alchemists) Antimony Soap Lead Gold Copper Arsenic Mercury Silver Iron Fire Oxygen Carbon Tin Water Nitrogen

JONS JAKOB BERZELIUS INVENTED THE PRESENT SYSTEM OF REPRESENTING THE ELEMENTS USNIG THE FIRST OR ANOTHER LETTER OF THEIR LATIN OR ENGLISH NAME. Symbols are written with the first letter in upper case.

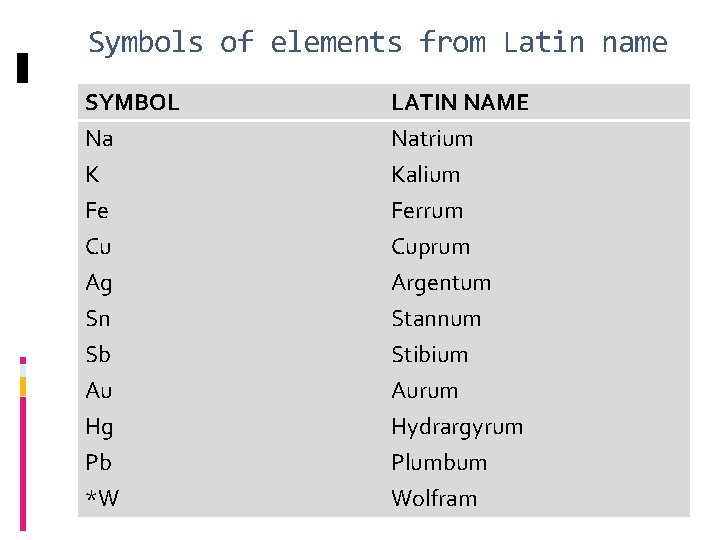
Symbols of elements from Latin name SYMBOL Na K Fe LATIN NAME Natrium Kalium Ferrum Cu Ag Sn Sb Au Hg Pb *W Cuprum Argentum Stannum Stibium Aurum Hydrargyrum Plumbum Wolfram

WAYS OF NAMING THE ELEMENTS From their discoverer/scientist – examples are Md, Lr, No, Es, Fm From the place of discovery – examples are Fr, Mg, Sc, Am, Bk, Eu From their properties – examples are

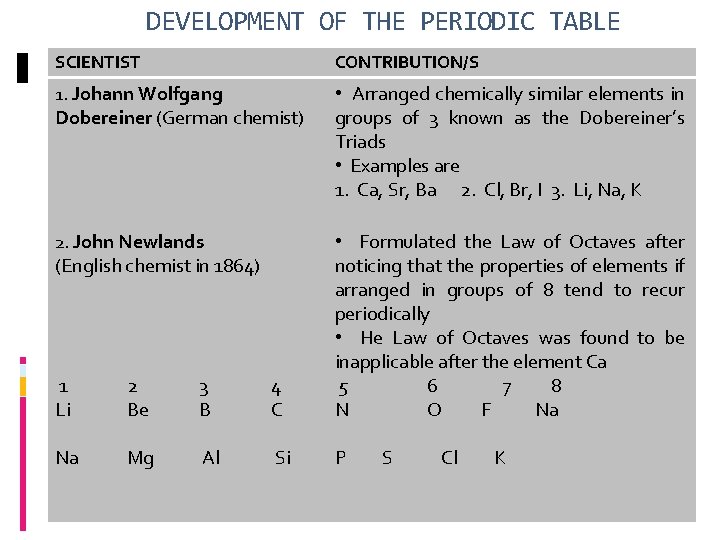
DEVELOPMENT OF THE PERIODIC TABLE SCIENTIST CONTRIBUTION/S 1. Johann Wolfgang • Arranged chemically similar elements in groups of 3 known as the Dobereiner’s Triads • Examples are 1. Ca, Sr, Ba 2. Cl, Br, I 3. Li, Na, K 2. John Newlands Dobereiner (German chemist) 1 Li 2 Be 3 B 4 C • Formulated the Law of Octaves after noticing that the properties of elements if arranged in groups of 8 tend to recur periodically • He Law of Octaves was found to be inapplicable after the element Ca 5 6 7 8 N O F Na Na Mg Al Si P (English chemist in 1864) S Cl K

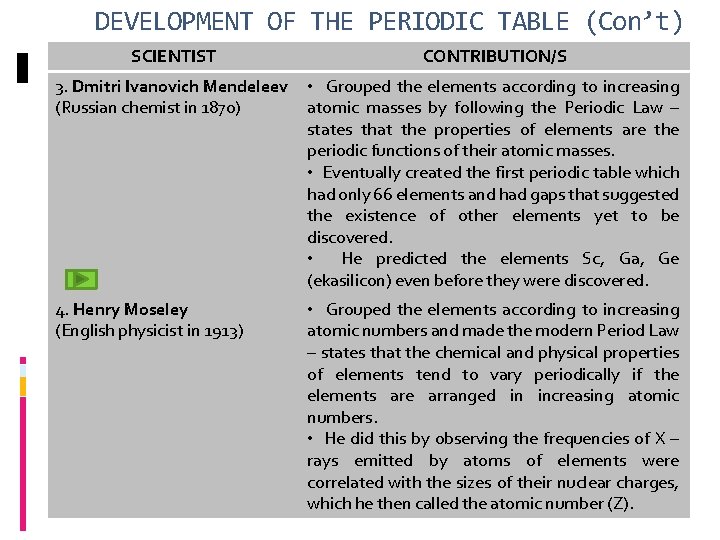
DEVELOPMENT OF THE PERIODIC TABLE (Con’t) SCIENTIST CONTRIBUTION/S 3. Dmitri Ivanovich Mendeleev (Russian chemist in 1870) • Grouped the elements according to increasing atomic masses by following the Periodic Law – states that the properties of elements are the periodic functions of their atomic masses. • Eventually created the first periodic table which had only 66 elements and had gaps that suggested the existence of other elements yet to be discovered. • He predicted the elements Sc, Ga, Ge (ekasilicon) even before they were discovered. 4. Henry Moseley (English physicist in 1913) • Grouped the elements according to increasing atomic numbers and made the modern Period Law – states that the chemical and physical properties of elements tend to vary periodically if the elements are arranged in increasing atomic numbers. • He did this by observing the frequencies of X – rays emitted by atoms of elements were correlated with the sizes of their nuclear charges, which he then called the atomic number (Z).

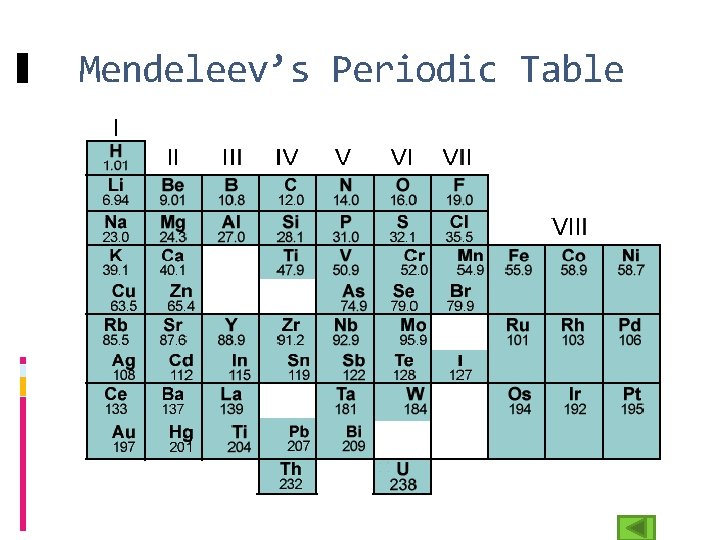
Mendeleev’s Periodic Table

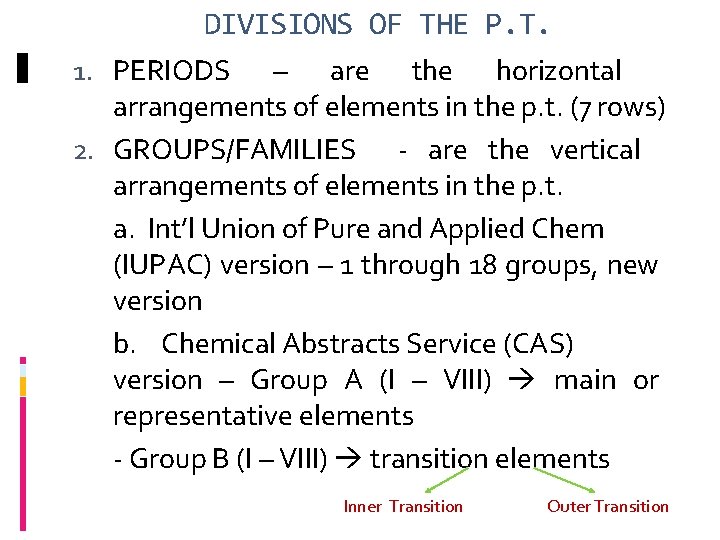
DIVISIONS OF THE P. T. 1. PERIODS – are the horizontal arrangements of elements in the p. t. (7 rows) 2. GROUPS/FAMILIES - are the vertical arrangements of elements in the p. t. a. Int’l Union of Pure and Applied Chem (IUPAC) version – 1 through 18 groups, new version b. Chemical Abstracts Service (CAS) version – Group A (I – VIII) main or representative elements - Group B (I – VIII) transition elements Inner Transition Outer Transition

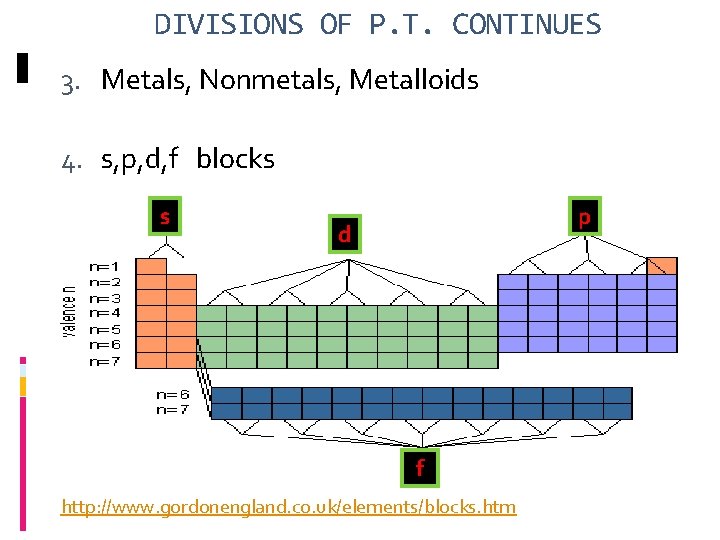
DIVISIONS OF P. T. CONTINUES 3. Metals, Nonmetals, Metalloids 4. s, p, d, f blocks s p d f http: //www. gordonengland. co. uk/elements/blocks. htm

4 Categories of Groups by Bohr 1. A Group – main/representative elements - composed of metals & nonmetals & metalloids where s & p sublevels are filled up partially or completely 2. Group O - Are nonmetals at the end of each period - Also known as the noble or inert gases; they are very unreactive - Have completely filled the s & p sublevels except He.

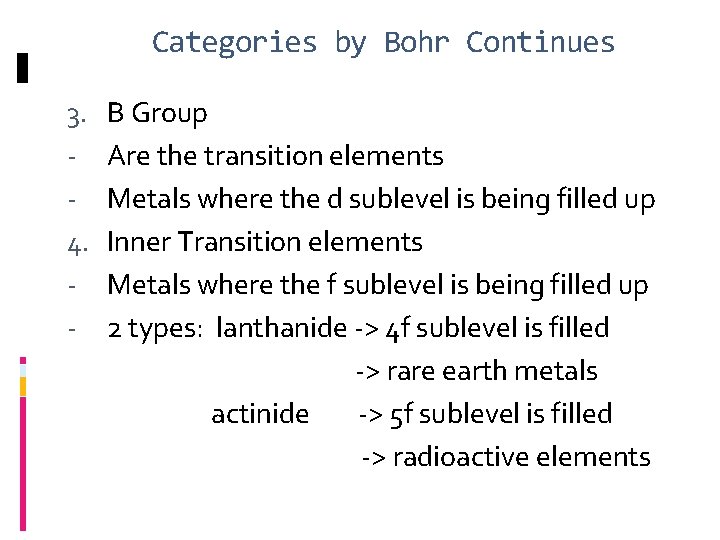
Categories by Bohr Continues 3. 4. - B Group Are the transition elements Metals where the d sublevel is being filled up Inner Transition elements Metals where the f sublevel is being filled up 2 types: lanthanide -> 4 f sublevel is filled -> rare earth metals actinide -> 5 f sublevel is filled -> radioactive elements

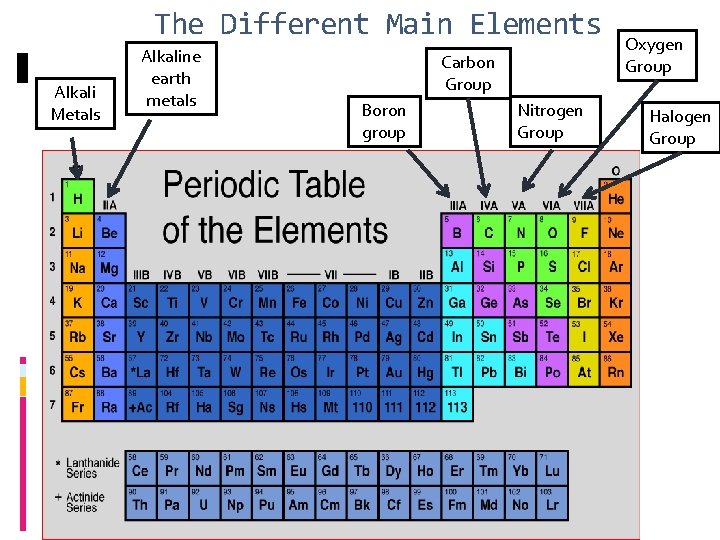
The Different Main Elements Alkali Metals Alkaline earth metals Carbon Group Boron group Nitrogen Group Oxygen Group Halogen Group